SUMMARY: Bones are the third most common site of metastatic disease and approximately 100,000 cases of bone metastasis are reported in the United States each year. Cancers originating in the breast, prostate, lung, thyroid and kidney, are more likely to metastasize to the bone. Bisphosphonates inhibit osteoclast-mediated bone resorption and both oral and IV bisphosphonates reduce the risk of developing Skeletal Related Events (SRE’s) and delay the time to SRE’s in patients with bone metastases. Bisphosphonates can also reduce bone pain and may improve Quality of life. Intravenous bisphosphonates, Pamidronate (AREDIA®) and Zoledronic acid (ZOMETA®) have been approved in the US for the treatment of bone metastases. Amino-bisphosphonate, ZOMETA® has however largely replaced AREDIA®, because of its superior efficacy. Both AREDIA® and ZOMETA® are administered IV every 3 to 4 weeks during the first year, following diagnoses of bone metastases. However, the optimal treatment schedule following this initial phase of treatment has remained unclear. Further, renal toxicity, long bone fractures and OsteoNecrosis of the Jaw (ONJ) have been identified as potential problems with bisphosphonate use.
Bisphosphonates can also reduce bone pain and may improve Quality of life. Intravenous bisphosphonates, Pamidronate (AREDIA®) and Zoledronic acid (ZOMETA®) have been approved in the US for the treatment of bone metastases. Amino-bisphosphonate, ZOMETA® has however largely replaced AREDIA®, because of its superior efficacy. Both AREDIA® and ZOMETA® are administered IV every 3 to 4 weeks during the first year, following diagnoses of bone metastases. However, the optimal treatment schedule following this initial phase of treatment has remained unclear. Further, renal toxicity, long bone fractures and OsteoNecrosis of the Jaw (ONJ) have been identified as potential problems with bisphosphonate use.
CALGB 70604 (Alliance), is a randomized phase III study in which the efficacy of ZOMETA® administered every 4 weeks was compared with ZOMETA® administered every 12 weeks, in patients with breast cancer, prostate cancer or multiple myeloma, with bone metastases. In this non-inferiority trial, 1822 patients (Breast = 833, Prostate = 674, Myeloma= 270 and Other= 45) were randomly assigned 1:1, to receive ZOMETA® every 4 weeks or every 12 weeks for 2 years. 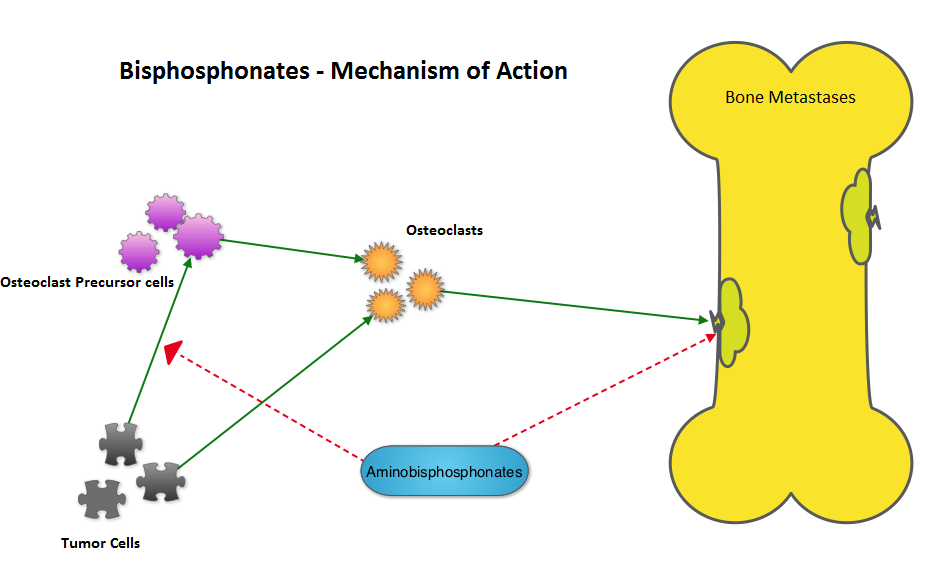 The primary endpoint was incidence of any Skeletal Related Event (SRE) and secondary endpoints included skeletal morbidity rates, performance status, pain using the Brief Pain Inventory and incidences of ONJ and renal dysfunction. Both treatment groups were well matched. Patients in this trial were stratified by disease and analyses by disease was pre-planned. It was noted that for the primary endpoint, there was no significant difference between the two treatment groups with 29% of patients in both treatment groups experiencing at least one SRE (P=0.79). With regards to secondary endpoints, there were still no significant differences between the two treatment groups, including renal dysfunction and ONJ. The authors pointed out that toxicities such as ONJ and renal dysfunction are more likely to occur after 2 years of treatment.
The primary endpoint was incidence of any Skeletal Related Event (SRE) and secondary endpoints included skeletal morbidity rates, performance status, pain using the Brief Pain Inventory and incidences of ONJ and renal dysfunction. Both treatment groups were well matched. Patients in this trial were stratified by disease and analyses by disease was pre-planned. It was noted that for the primary endpoint, there was no significant difference between the two treatment groups with 29% of patients in both treatment groups experiencing at least one SRE (P=0.79). With regards to secondary endpoints, there were still no significant differences between the two treatment groups, including renal dysfunction and ONJ. The authors pointed out that toxicities such as ONJ and renal dysfunction are more likely to occur after 2 years of treatment.
It was concluded that ZOMETA® administered every 3 months for 2 years is non-inferior to ZOMETA® administered every 4 weeks for 2 years, in patients with breast cancer, prostate cancer and multiple myeloma, with bone metastases. A less frequent dosing of ZOMETA® compared with the standard monthly dosing, may be more convenient for the patients and cost effective. CALGB 70604 (Alliance): A randomized phase III study of standard dosing vs. longer interval dosing of zoledronic acid in metastatic cancer. Himelstein AL, Qin R, Novotny PJ, et al. J Clin Oncol 33, 2015 (suppl; abstr 9501)

