SUMMARY: The FDA on August 25, 2021, approved TIBSOVO® (Ivosidenib) for adult patients with previously treated, locally advanced or metastatic Cholangiocarcinoma, with an Isocitrate DeHydrogenase-1 (IDH1) mutation, as detected by an FDA-approved test. The FDA also approved the Oncomine Dx Target Test (Life Technologies Corporation) as a companion diagnostic device, to aid in selecting patients with Cholangiocarcinoma for treatment with TIBSOVO®.
Bile Duct cancer (Cholangiocarcinoma), comprise about 30% of all primary liver tumors and includes both intrahepatic and extrahepatic bile duct cancers. Klatskin tumor is a type of Cholangiocarcinoma that begins in the hilum, at the junction of the left and right bile ducts. It is the most common type of Cholangiocarcinoma, accounting for more than half of all cases. About 8,000 people in the US are diagnosed with Cholangiocarcinoma each year and approximately 20% of the cases are suitable for surgical resection. The 5-year survival is less than 10%, with limited progress made over the past two decades. There is therefore an unmet need for new effective therapies.
Isocitrate DeHydrogenase (IDH) is a metabolic enzyme that helps generate energy from glucose and other metabolites, by catalyzing the conversion of Isocitrate to Alpha-Ketoglutarate. Alpha-ketoglutarate is required to properly regulate DNA and histone methylation, which in turn is important for gene expression and cellular differentiation. IDH mutations lead to aberrant DNA methylation and altered gene expression thereby preventing cellular differentiation, with resulting immature undifferentiated cells. IDH mutations can thus promote leukemogenesis in Acute Myeloid Leukemia (AML) and tumorigenesis in solid tumors and can result in inferior outcomes. There are three isoforms of IDH. IDH1 is mainly found in the cytoplasm, as well as in peroxisomes, whereas IDH2 and IDH3 are found in the mitochondria, and are a part of the Krebs cycle. Approximately 20% of patients with AML, 70% of patients with Low-grade Glioma and secondary Glioblastoma, 50% of patients with Chondrosarcoma, 20% of patients with Intrahepatic Cholangiocarcinoma, 30% of patients with Angioimmunoblastic T-cell lymphoma and 8% of patients with Myelodysplastic syndromes/Myeloproliferative neoplasms, are associated with IDH mutations.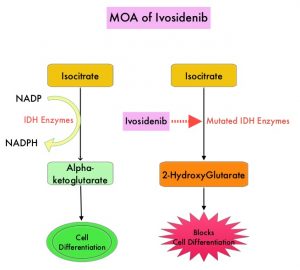
TIBSOVO® (Ivosidenib) is an oral, targeted, small-molecule inhibitor of mutant IDH1. A previously published Phase I study demonstrated the safety and activity of TIBSOVO® in patients with IDH1 mutated advanced Cholangiocarcinoma. ClarIDHy is an international, randomized, double-blind, Phase III study, in which 187 previously treated patients with advanced Cholangiocarcinoma with an IDH1 mutation were randomly assigned 2:1 to receive TIBSOVO® 500 mg orally once daily (N=126) or matched placebo (N=61). All patients had advanced unresectable Cholangiocarcinoma. The median age was 62 years, 91% had intrahepatic Cholangiocarcinoma, 93% of patients had metastatic disease and 47% had received two prior therapies. The patient’s disease must have progressed following at least one, but not more than two prior regimens, including at least one Gemcitabine or 5-FU containing regimen. The Primary endpoint was Progression Free Survival (PFS) and Secondary endpoints included Safety, Objective Response Rate (ORR) and Overall Survival (OS). Crossover from placebo to TIBSOVO® was permitted upon radiographic disease progression.
This study met its Primary endpoint and the median PFS was 2.7 months for patients treated with TIBSOVO® compared to 1.4 months with placebo (HR=0.37; P<0.0001). More importantly, the median PFS at 6 and 12 months were 32% and 22% in the TIBSOVO® group, whereas no patients randomized to the placebo group were progression-free for 6 or more months, at the time of data cutoff.
The authors also reported the results of final analysis which showed an improvement in the secondary endpoint of OS, favoring patients randomized to TIBSOVO® compared to those randomized to placebo. However, statistical significance was not reached. The median OS for patients in the TIBSOVO® arm was 10.3 months compared to 7.5 months for patients in the placebo arm (HR=0.79; 1-sided P=0.093). A high proportion of patients in the placebo arm (70.5%) crossed over to TIBSOVO®. After adjusting for crossover from placebo to TIBSOVO®, the median OS for patients in the placebo arm was 5.1 months (HR=0.49; 1-sided P<0.0001).
The 6-month survival rate for patients in the TIBSOVO® arm was 69% compared to 57% of patients in the placebo arm, not adjusted for crossover. The 12-month survival rate for patients in the TIBSOVO® arm was 43% compared to 36% for patients in the placebo arm, not adjusted for crossover. Treatment with TIBSOVO® preserved patients’ physical functioning from baseline, as assessed by the EORTC QLQ-C30 questionnaire, whereas patients in the placebo arm experienced decline from baseline starting cycle 2. The most common Adverse Events of any grade for TIBSOVO® were nausea (38%), diarrhea (33.1%) and fatigue (28.9%). Adverse Events leading to discontinuation were more common with placebo compared with total TIBSOVO® (8.5% versus 6.6%).
It was concluded that treatment with TIBSOVO® in patients with advanced Cholangiocarcinoma with an IDH1 mutation, resulted in significant improvement in Progression Free Survival as well as favorable Overall Survival trend, when compared to Placebo, despite a high rate of crossover. This is the first pivotal study demonstrating the clinical benefit of targeting IDH1 mutation in this patient group. This new oral, non-cytotoxic, targeted treatment option, with a tolerable safety profile, will be a welcome addition to treat this aggressive disease, for which there is an unmet need for new therapies.
Final results from ClarIDHy, a global, phase III, randomized, double-blind study of ivosidenib (IVO) versus placebo (PBO) in patients (pts) with previously treated cholangiocarcinoma (CCA) and an isocitrate dehydrogenase 1 (IDH1) mutation. Zhu A, Macarulla T, Javle MM, et al. J Clin Oncol 39, 2021 (suppl 3; abstr 266)

