SUMMARY: The FDA on August 10, 2021, approved the combination of LENVIMA® (Lenvatinib) plus KEYTRUDA® (Pembrolizumab) for first line treatment of adult patients with advanced Renal Cell Carcinoma (RCC). The American Cancer Society estimates that 76,080 new cases of kidney cancers will be diagnosed in the United States in 2021 and about 13,780 people will die from the disease. Renal Cell Carcinoma (RCC) is by far the most common type of kidney cancer and is about twice as common in men as in women. Modifiable risk factors include smoking, obesity, workplace exposure to certain substances and high blood pressure. The five year survival of patients with advanced RCC is less than 10% and there is a significant unmet need for improved therapies for this disease.
SUTENT® (Sunitinib) is a MultiKinase Inhibitor (MKI) which simultaneously targets the tumor cell wall, vascular endothelial cell wall as well as the pericyte/fibroblast/vascular/smooth vessel cell wall, and is capable of specifically binding to tyrosine kinases inhibiting the earlier signaling events and thereby inhibits phosphorylation of VEGF receptor, PDGF receptor, FLT-3 and c-KIT. SUTENT® has been the standard first line intervention for treatment naïve patients with advanced RCC. In a large, multi-center, randomized, Phase III study, the median Progression Free Survival (PFS) with SUTENT® was 9.5 months, the Objective Response Rate (ORR) was 25%, and the median Overall Survival (OS) was 29.3 months, when compared with Interferon Alfa, in patients with treatment-naïve Renal Cell Carcinoma. This was however associated with a high rate of hematological toxicities.
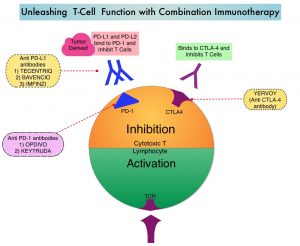
KEYTRUDA® (Pembrolizumab) is a fully humanized, Immunoglobulin G4, anti-PD-1, monoclonal antibody, that binds to the PD-1 receptor and blocks its interaction with ligands PD-L1 and PD-L2. It thereby reverses the PD-1 pathway-mediated inhibition of the immune response and unleashes the tumor-specific effector T cells.
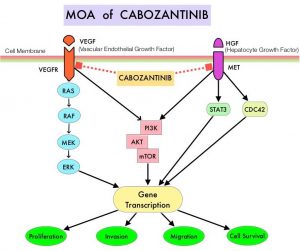
LENVIMA® (Lenvatinib) is an oral multitargeted TKI which targets Vascular Endothelial Growth Factor Receptor (VEGFR) 1-3, Fibroblast Growth Factor Receptor (FGFR) 1-4, Rearranged during Transfection tyrosine kinase receptor (RET), c-KIT, and Platelet Derived Growth Factor Receptor (PDGFR). LENVIMA® differs from other TKIs with antiangiogenesis properties by its ability to inhibit FGFR-1, thereby blocking the mechanisms of resistance to VEGF/VEGFR inhibitors. In addition, it controls tumor cell growth by inhibiting RET, c-KIT, and PDGFR beta and influences tumor microenvironment by inhibiting FGFR and PDGFR beta.
AFINITOR® (Everolimus) does not inhibit tyrosine kinases, but is a specific inhibitor of mTOR (Mammalian Target of Rapamycin), which is a serine/threonine kinase, normally activated further downstream in the signaling cascade. With the inhibition of mTOR, protein synthesis is inhibited resulting in decreased angiogenesis, cell proliferation and survival as well as decreased levels of HIF-1 alpha.
A combination of LENVIMA® plus AFINITOR® was shown to be associated with longer Progression Free Survival than AFINITOR® alone as second line treatment in advanced RCC (Lancet Oncol 2015;16:1473-1482). LENVIMA® plus KEYTRUDA® was shown to have promising antitumor activity in previously treated patients with RCC in a Phase IB-II trial (J Clin Oncol 2020;38:1154-1163). Based on this data, the authors conducted a multicenter, randomized, open-label, Phase III trial to compare the efficacy and safety of LENVIMA® in combination with KEYTRUDA® or AFINITOR® versus SUTENT® alone, in first line treatment of patients with advanced RCC.
The researchers randomly assigned 1069 patients with advanced RCC and no previous systemic therapy in a 1:1:1 ratio to receive LENVIMA® 20 mg orally once daily plus KEYTRUDA® 200 mg IV once every 3 weeks (N=355), LENVIMA® 18 mg orally once daily plus AFINITOR® 5 mg orally once daily (N=357) or SUTENT® 50 mg orally once daily, alternating 4 weeks on and 2 weeks off (N=357). The Primary end point was Progression Free Survival (PFS) and Secondary endpoints included Overall Survival (OS), Objective Response Rate (ORR) and Safety. The median follow up for OS was 26.6 months.
The median PFS was significantly longer with LENVIMA® plus KEYTRUDA® combination, compared to single agent SUTENT® (23.9 months versus 9.2 months, HR=0.39; P<0.001). The median PFS with the LENVIMA® plus AFINITOR® combination was also significantly longer, compared to single agent SUTENT® (14.7 months versus 9.2 months, HR=0.65; P<0.001). The PFS benefit favored the two LENVIMA® combination regimens over single agent SUTENT® across all evaluated subgroups, including those based on MSKCC prognostic risk group and International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) risk group. At interim analysis, the OS was significantly longer with LENVIMA® plus KEYTRUDA® than with SUTENT® (HR for death=0.66; P=0.005). This benefit was noted in most subgroups, including patients with PD-L1 positive or negative tumors, with an exception of patients with favorable risk disease as defined by IMDC criteria. Overall Survival with LENVIMA® plus AFINITOR® was however not significantly longer compared with SUTENT® (HR=1.15; P=0.30).
The confirmed ORR was 71% with LENVIMA® plus KEYTRUDA®, 53.5% with LENVIMA® plus AFINITOR®, and 36.1% with single agent SUTENT®. The Complete Response rate was 16.1% in the LENVIMA® plus KEYTRUDA® group, 9.8% in the LENVIMA® plus AFINITOR® group, and 4.2% in the SUTENT® group. The median Duration of Response in patients who had a confirmed response was 25.8 months in the LENVIMA® plus KEYTRUDA® group, 16.6 months in the LENVIMA® plus AFINITOR® group, and 14.6 months in the SUTENT® group. Grade 3 or higher Adverse Events occurred in 82.4% of the patients who received LENVIMA® plus KEYTRUDA® group, in 83.1% of the patients who received LENVIMA® plus AFINITOR®, and in 71.8% of the patients who received SUTENT®.
It was concluded that a combination of LENVIMA® plus KEYTRUDA® provided superior Progression Free Survival and Overall Survival compared to SUTENT®, in the first line treatment of patients with advanced Renal Cell Carcinoma.
Lenvatinib plus Pembrolizumab or Everolimus for Advanced Renal Cell Carcinoma. Motzer R, Alekseev B, Rha S-Y, et al. for the CLEAR Trial Investigators. N Engl J Med 2021; 384:1289-1300