SUMMARY: Prostate cancer is the most common cancer in American men excluding skin cancer and 1 in 7 men will be diagnosed with prostate cancer during their lifetime. It is estimated that in the United States, about 220,800 new cases of prostate cancer will be diagnosed in 2015 and over 27,000 men will die of the disease. The major source of PSA (Prostate Specific Antigen) is the prostate gland and the PSA levels are therefore undetectable within 6 weeks after Radical Prostatectomy. Similarly, following Radiation Therapy, there is a gradual decline in PSA before reaching a post treatment nadir. A detectable PSA level after Radical Prostatectomy, or a rising PSA level following Radiation Therapy, is considered PSA failure or biochemical recurrence.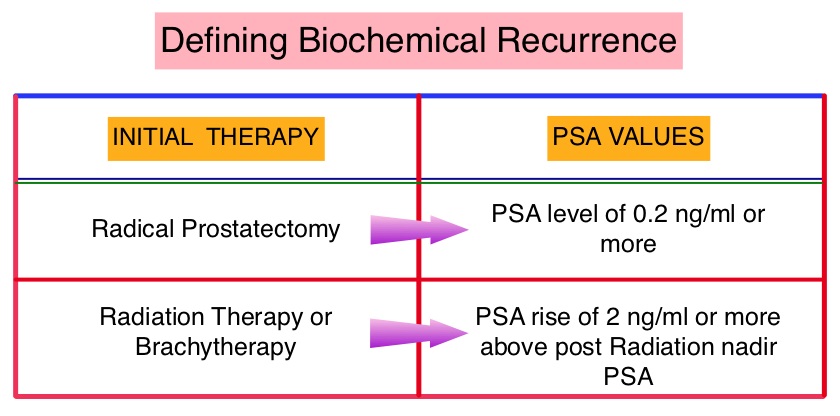 The American Urological Association suggested that a PSA of 0.2 ng/mL or higher after Radical Prostatectomy, defines PSA failure or relapse. A PSA rise of 2 ng/ml or more above post Radiation Therapy nadir is considered PSA failure or relapse. Approximately 35% of the patients with prostate cancer will experience PSA only relapse within 10 years of their primary treatment and a third of these patients will develop documented metastatic disease within 8 years following PSA only relapse. The development and progression of prostate cancer is driven by androgens. Androgen Deprivation Therapy (ADT) has therefore been the cornerstone of treatment of advanced prostate cancer and is the first treatment intervention for hormone sensitive prostate cancer. The appropriate time (immediate versus delayed) to start Androgen Deprivation Therapy (ADT) in patients with prostate cancer with rising Prostate-Specific Antigen (PSA), as the only sign of relapse, has remained unclear. This has been partly due to lack of patient accruals and patient reluctance to be randomized, in these clinical trials.
The American Urological Association suggested that a PSA of 0.2 ng/mL or higher after Radical Prostatectomy, defines PSA failure or relapse. A PSA rise of 2 ng/ml or more above post Radiation Therapy nadir is considered PSA failure or relapse. Approximately 35% of the patients with prostate cancer will experience PSA only relapse within 10 years of their primary treatment and a third of these patients will develop documented metastatic disease within 8 years following PSA only relapse. The development and progression of prostate cancer is driven by androgens. Androgen Deprivation Therapy (ADT) has therefore been the cornerstone of treatment of advanced prostate cancer and is the first treatment intervention for hormone sensitive prostate cancer. The appropriate time (immediate versus delayed) to start Androgen Deprivation Therapy (ADT) in patients with prostate cancer with rising Prostate-Specific Antigen (PSA), as the only sign of relapse, has remained unclear. This has been partly due to lack of patient accruals and patient reluctance to be randomized, in these clinical trials.
The authors conducted this randomized, prospective, phase III trial, to determine if immediate intervention with Androgen Deprivation Therapy (ADT) would improve Overall Survival (OS), compared with delayed ADT, in prostate cancer patients with PSA relapse, following definitive therapy, or in asymptomatic men not suitable for definitive therapy at the time of diagnosis. This analysis combined prostate cancer patients with PSA relapse enrolled in two separate studies. Two hundred and ninety three (N=293) eligible patients were randomly assigned 1:1 to immediate Androgen Deprivation Therapy (N= 142) or delayed ADT (N=151). The primary endpoint was unadjusted Overall Survival. Secondary endpoints included cancer-specific survival and time to clinical progression. The median follow up was 5 years. There was a statistically significant improvement in the Overall Survival, with a 45% reduction in the risk for death, for those receiving immediate ADT compared with the delayed treatment group (HR=0.55; P=0.05). Further, with immediate ADT, there was a statistically significant delay in the time to first local progression (HR= 0.51; P=0.001) as well as time to first metastatic disease (HR=0.54; P=0.018). The authors concluded that immediate Androgen Deprivation Therapy significantly improved Overall Survival and time to clinical progression for prostate cancer patients with PSA relapse, after definitive therapy. This benefit however must be weighed against the risks associated with long term Androgen Deprivation Therapy. TROG 03.06 and VCOG PR 01-03: The “Timing Of Androgen Deprivation therapy in prostate cancer patients with a rising PSA (TOAD)” collaborative randomised phase III trial. Duchesne GM, Bassett J, D’Este C, et al. J Clin Oncol 33, 2015 (suppl; abstr 5007)

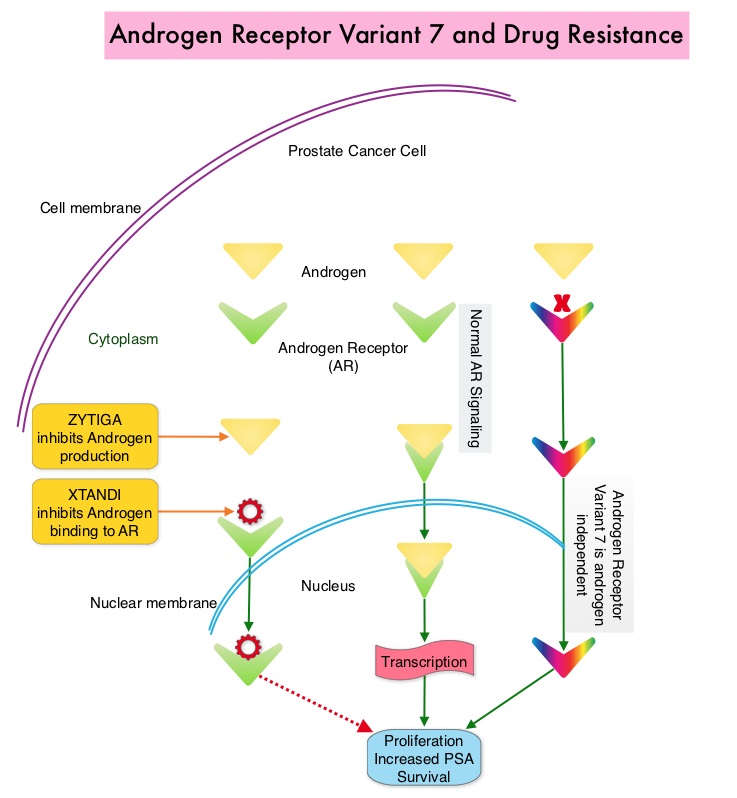 Both these agents have been shown to improve survival in metastatic CRPC. ZYTIGA® inhibits CYP 17A1 enzyme and depletes adrenal and intratumoral androgens, thereby impairing AR signaling. XTANDI® competes with Testosterone and Dihydrotestosterone and avidly binds to the Androgen Receptor, thereby inhibiting AR signaling and in addition inhibits translocation of the AR into the nucleus and thus inhibits the transcriptional activities of the AR. About 20-40% of the patients do not respond to these newer agents and even those who respond will invariably develop resistance to these drugs. This again has been attributed to persistent AR signaling by variant forms of Androgen Receptor, generated through somatic mutation or aberrant RNA splicing. Androgen Receptor Variant AR-V7 can be detected in the CTCs (Circulating Tumor Cells). AR-V7 does not have the domain to bind androgens and may be associated with resistance to XTANDI®. Further AR-V7 is constitutively active and can independently activate transcription factors and therefore is not effected by androgen depleting agents including ZYTIGA®. With this background, the authors hypothesized that detection of Androgen Receptor variant AR-V7 in circulating tumor cells from men with metastatic prostate cancer would be associated with resistance to both ZYTIGA® and XTANDI®. In a previously published prospective study, data involving 62 patients showed that detection of AR-V7 in Circulating Tumor Cells (CTCs) in men with mCRPC was indeed associated with primary resistance to both ZYTIGA® and XTANDI®. AR-V7–positive patients had inferior overall survival with both XTANDI® (HR = 6.9; P =0.002) and ZYTIGA® (HR = 12.7; P =0.006). AR-V7 was therefore shown to have a prognostic value for outcomes in mCRPC with ZYTIGA® and XTANDI®. In this present publication, the authors studied to determine if AR-V7-positive patients would retain sensitivity to Taxane chemotherapy. The researchers in this small prospective study enrolled 37 patients with metastatic CRPC who were starting Taxane chemotherapy with Cabazitaxel (JEVTANA®) or Docetaxel (TAXOTERE®). Presence or lack of AR-V7 in circulating tumor cells (CTCs), was determined by the qRT-PCR assay. Of the enrolled patients, 46% had detectable AR-V7 in CTCs. The primary endpoint was associations between AR-V7 status and PSA response rates and secondary endpoints included Progression Free Survival (PFS). They noted that the PSA responses were achieved in both AR-V7- positive and AR-V7-negative men and the difference was non-significant (41% versus 65%, P=0.19). Likewise there was no significant difference in the median PFS in AR-V7-positive and AR-V7-negative men (5.1 versus 6.9 months (HR= 2.65; P=0.11). The researchers then combined the data from their previously published study with 62 patients and they noted that, in AR-V7-positive men, PSA responses were higher in Taxane treated versus ZYTIGA®/XTANDI® treated men (41% versus 0%, P<0.001) and PFS were longer in the Taxane treated men as well (HR for PFS = 0.21, P=0.003). The outcomes however did not differ by treatment type in AR-V7-negative men and were comparable. The authors concluded that detection of AR-V7 in CTCs from men with mCRPC is not associated with primary resistance to Taxane chemotherapy, and such patients may retain sensitivity to Taxanes. In AR-V7-positive men however, Taxanes appear to be more efficacious than ZYTIGA® or XTANDI®. AR-V7 once available commercially, may become a biomarker for treatment selection, in metastatic Castrate Resistant Prostate Cancer. AR splice variant 7 (AR-V7) and response to taxanes in men with metastatic castration-resistant prostate cancer (mCRPC). Antonarakis ES, Lu C, Chen Y, et al. J Clin Oncol 33, 2015 (suppl 7; abstr 138)
Both these agents have been shown to improve survival in metastatic CRPC. ZYTIGA® inhibits CYP 17A1 enzyme and depletes adrenal and intratumoral androgens, thereby impairing AR signaling. XTANDI® competes with Testosterone and Dihydrotestosterone and avidly binds to the Androgen Receptor, thereby inhibiting AR signaling and in addition inhibits translocation of the AR into the nucleus and thus inhibits the transcriptional activities of the AR. About 20-40% of the patients do not respond to these newer agents and even those who respond will invariably develop resistance to these drugs. This again has been attributed to persistent AR signaling by variant forms of Androgen Receptor, generated through somatic mutation or aberrant RNA splicing. Androgen Receptor Variant AR-V7 can be detected in the CTCs (Circulating Tumor Cells). AR-V7 does not have the domain to bind androgens and may be associated with resistance to XTANDI®. Further AR-V7 is constitutively active and can independently activate transcription factors and therefore is not effected by androgen depleting agents including ZYTIGA®. With this background, the authors hypothesized that detection of Androgen Receptor variant AR-V7 in circulating tumor cells from men with metastatic prostate cancer would be associated with resistance to both ZYTIGA® and XTANDI®. In a previously published prospective study, data involving 62 patients showed that detection of AR-V7 in Circulating Tumor Cells (CTCs) in men with mCRPC was indeed associated with primary resistance to both ZYTIGA® and XTANDI®. AR-V7–positive patients had inferior overall survival with both XTANDI® (HR = 6.9; P =0.002) and ZYTIGA® (HR = 12.7; P =0.006). AR-V7 was therefore shown to have a prognostic value for outcomes in mCRPC with ZYTIGA® and XTANDI®. In this present publication, the authors studied to determine if AR-V7-positive patients would retain sensitivity to Taxane chemotherapy. The researchers in this small prospective study enrolled 37 patients with metastatic CRPC who were starting Taxane chemotherapy with Cabazitaxel (JEVTANA®) or Docetaxel (TAXOTERE®). Presence or lack of AR-V7 in circulating tumor cells (CTCs), was determined by the qRT-PCR assay. Of the enrolled patients, 46% had detectable AR-V7 in CTCs. The primary endpoint was associations between AR-V7 status and PSA response rates and secondary endpoints included Progression Free Survival (PFS). They noted that the PSA responses were achieved in both AR-V7- positive and AR-V7-negative men and the difference was non-significant (41% versus 65%, P=0.19). Likewise there was no significant difference in the median PFS in AR-V7-positive and AR-V7-negative men (5.1 versus 6.9 months (HR= 2.65; P=0.11). The researchers then combined the data from their previously published study with 62 patients and they noted that, in AR-V7-positive men, PSA responses were higher in Taxane treated versus ZYTIGA®/XTANDI® treated men (41% versus 0%, P<0.001) and PFS were longer in the Taxane treated men as well (HR for PFS = 0.21, P=0.003). The outcomes however did not differ by treatment type in AR-V7-negative men and were comparable. The authors concluded that detection of AR-V7 in CTCs from men with mCRPC is not associated with primary resistance to Taxane chemotherapy, and such patients may retain sensitivity to Taxanes. In AR-V7-positive men however, Taxanes appear to be more efficacious than ZYTIGA® or XTANDI®. AR-V7 once available commercially, may become a biomarker for treatment selection, in metastatic Castrate Resistant Prostate Cancer. AR splice variant 7 (AR-V7) and response to taxanes in men with metastatic castration-resistant prostate cancer (mCRPC). Antonarakis ES, Lu C, Chen Y, et al. J Clin Oncol 33, 2015 (suppl 7; abstr 138)
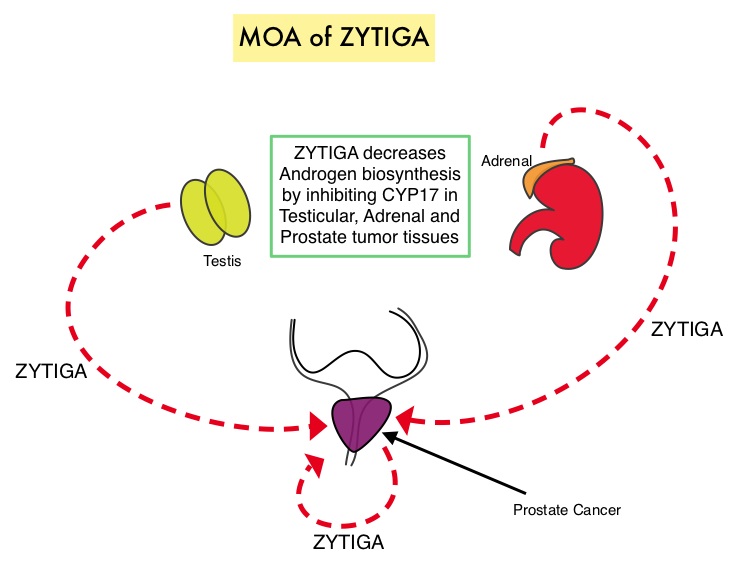 Abiraterone acetate (ZYTIGA®) is a novel, targeted, oral androgen biosynthesis inhibitor that decreases androgen production in the adrenal glands, testes and prostate cancer cells by inhibiting a steroidal enzyme CYP17A. COU-AA-302 trial is a placebo controlled, double-blind, randomized, phase III study and at the interim analyses showed that ZYTIGA® plus Prednisone significantly improved radiographic Progression Free Survival compared with placebo plus Prednisone, in men with chemotherapy-naive CRPC. The authors in this publication provided additional data on the pre-specified final analysis of the trial, which included the effect of ZYTIGA® plus Prednisone on Overall Survival, time to opiate use, and use of other subsequent therapies. In this study which included 1088 chemotherapy-naïve, asymptomatic or mildly symptomatic CRPC patients, 546 patients received ZYTIGA® 1000 mg PO plus Prednisone 5 mg PO twice daily and 542 patients received placebo plus Prednisone. The co-primary endpoints were radiographic Progression Free Survival and Overall Survival. In the final analysis, at a median follow up of 49•2 months, the median Overall Survival was 34.7 months in the ZYTIGA® group vs 30.3 months in the placebo control group (HR = 0.81, P = 0.0033). This benefit was seen across all prespecified subgroups, as well as adjusting for crossover from placebo to ZYTIGA® (HR = 0.79, P = 0.0013). The median time to opiate use for cancer-related pain was significantly prolonged in the ZYTIGA® group compared to the placebo group (33.4 vs 23.4 months, HR = 0.72, P < 0.0001). The most common grade 3 or 4 adverse events of special interest in the ZYTIGA® vs placebo group were cardiac disorders (8% vs 4%), increased ALT (6% vs < 1%), and hypertension (5% vs 3%). The authors concluded that with a median follow up of more than 4 years, treatment with ZYTIGA® in patients with chemotherapy-naive metastatic CRPC, significantly improved Overall Survival compared with Prednisone alone, with favorable toxicities. Ryan CJ, Smith MR, Fizazi K, et al. for the COU-AA-302 Investigators .The Lancet Oncology 2015; 16:152-160
Abiraterone acetate (ZYTIGA®) is a novel, targeted, oral androgen biosynthesis inhibitor that decreases androgen production in the adrenal glands, testes and prostate cancer cells by inhibiting a steroidal enzyme CYP17A. COU-AA-302 trial is a placebo controlled, double-blind, randomized, phase III study and at the interim analyses showed that ZYTIGA® plus Prednisone significantly improved radiographic Progression Free Survival compared with placebo plus Prednisone, in men with chemotherapy-naive CRPC. The authors in this publication provided additional data on the pre-specified final analysis of the trial, which included the effect of ZYTIGA® plus Prednisone on Overall Survival, time to opiate use, and use of other subsequent therapies. In this study which included 1088 chemotherapy-naïve, asymptomatic or mildly symptomatic CRPC patients, 546 patients received ZYTIGA® 1000 mg PO plus Prednisone 5 mg PO twice daily and 542 patients received placebo plus Prednisone. The co-primary endpoints were radiographic Progression Free Survival and Overall Survival. In the final analysis, at a median follow up of 49•2 months, the median Overall Survival was 34.7 months in the ZYTIGA® group vs 30.3 months in the placebo control group (HR = 0.81, P = 0.0033). This benefit was seen across all prespecified subgroups, as well as adjusting for crossover from placebo to ZYTIGA® (HR = 0.79, P = 0.0013). The median time to opiate use for cancer-related pain was significantly prolonged in the ZYTIGA® group compared to the placebo group (33.4 vs 23.4 months, HR = 0.72, P < 0.0001). The most common grade 3 or 4 adverse events of special interest in the ZYTIGA® vs placebo group were cardiac disorders (8% vs 4%), increased ALT (6% vs < 1%), and hypertension (5% vs 3%). The authors concluded that with a median follow up of more than 4 years, treatment with ZYTIGA® in patients with chemotherapy-naive metastatic CRPC, significantly improved Overall Survival compared with Prednisone alone, with favorable toxicities. Ryan CJ, Smith MR, Fizazi K, et al. for the COU-AA-302 Investigators .The Lancet Oncology 2015; 16:152-160
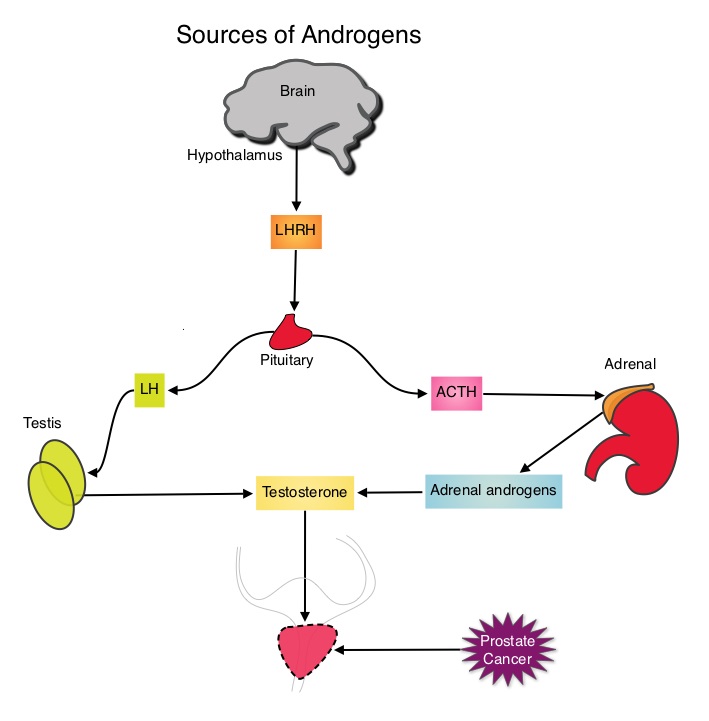 The development and progression of prostate cancer is driven by androgens. Androgen Deprivation Therapy (ADT) has therefore been the cornerstone of treatment of advanced prostate cancer and is the first treatment intervention for hormone sensitive prostate cancer. Chemotherapy is usually considered for patients who progress on hormone therapy and TAXOTERE® (Docetaxel) has been shown to improve Overall Survival (OS) of metastatic prostate cancer patients, who had progressed on androgen deprivation therapy. It is not clear however, whether ADT is more effective with or without TAXOTERE®, when treating patients with metastatic prostate cancer. To address this further, a randomized phase III trial (E3805) was conducted to assess the benefit of upfront treatment with a combination of chemotherapy and hormonal therapy, in patients with metastatic hormone sensitive prostate cancer. Seven hundred and ninety (N=790) patients with newly diagnosed metastatic prostate cancer were randomly assigned to receive either Androgen Deprivation Therapy alone (N=393) or ADT plus TAXOTERE® (N=397). Androgen Deprivation Therapy consisted of either Luteinizing Hormone Releasing Hormone (LHRH) agonist therapy, LHRH antagonist therapy, or surgical castration. Chemotherapy consisted of TAXOTERE®, started within 4 months of starting ADT, dosed at 75 mg/m2 given every 3 weeks for a maximum of six cycles. The median age of patients was 63 years and approximately two-thirds of patients had high-volume disease, with either extensive liver or bone metastases. The primary endpoint of this study was Overall Survival. At a median follow up of 29 months, the median Overall Survival was 42.3 months in the ADT group and 52.7 months in the ADT plus TAXOTERE® group (HR=0.63; P<0.0006). This benefit was even more significant in patients with high volume disease (32.2 vs 49.2 months for ADT and ADT plus TAXOTERE® respectively, HR=0.62; P<0.0012). At 12 months, the proportion of patients with PSA levels less than 0.2 ng/mL was 9.4% in the ADT alone group vs 19.7% in the ADT plus TAXOTERE® group (P < 0.0001). The median time to clinical progression was 19.8 months in the ADT alone group vs 32.7 months in the ADT plus TAXOTERE® group (P < 0.0001). The authors concluded that this is the first study to demonstrate survival benefit in patients with newly diagnosed metastatic prostate cancer. This survival benefit with Androgen Deprivation Therapy and TAXOTERE® is even more so, in patients with high volume disease and should be considered standard treatment for those patients who are fit to receive TAXOTERE® based therapy. Sweeney C, Chen Y, Carducci MA, et al. 2014 ASCO Annual Meeting; LBA2
The development and progression of prostate cancer is driven by androgens. Androgen Deprivation Therapy (ADT) has therefore been the cornerstone of treatment of advanced prostate cancer and is the first treatment intervention for hormone sensitive prostate cancer. Chemotherapy is usually considered for patients who progress on hormone therapy and TAXOTERE® (Docetaxel) has been shown to improve Overall Survival (OS) of metastatic prostate cancer patients, who had progressed on androgen deprivation therapy. It is not clear however, whether ADT is more effective with or without TAXOTERE®, when treating patients with metastatic prostate cancer. To address this further, a randomized phase III trial (E3805) was conducted to assess the benefit of upfront treatment with a combination of chemotherapy and hormonal therapy, in patients with metastatic hormone sensitive prostate cancer. Seven hundred and ninety (N=790) patients with newly diagnosed metastatic prostate cancer were randomly assigned to receive either Androgen Deprivation Therapy alone (N=393) or ADT plus TAXOTERE® (N=397). Androgen Deprivation Therapy consisted of either Luteinizing Hormone Releasing Hormone (LHRH) agonist therapy, LHRH antagonist therapy, or surgical castration. Chemotherapy consisted of TAXOTERE®, started within 4 months of starting ADT, dosed at 75 mg/m2 given every 3 weeks for a maximum of six cycles. The median age of patients was 63 years and approximately two-thirds of patients had high-volume disease, with either extensive liver or bone metastases. The primary endpoint of this study was Overall Survival. At a median follow up of 29 months, the median Overall Survival was 42.3 months in the ADT group and 52.7 months in the ADT plus TAXOTERE® group (HR=0.63; P<0.0006). This benefit was even more significant in patients with high volume disease (32.2 vs 49.2 months for ADT and ADT plus TAXOTERE® respectively, HR=0.62; P<0.0012). At 12 months, the proportion of patients with PSA levels less than 0.2 ng/mL was 9.4% in the ADT alone group vs 19.7% in the ADT plus TAXOTERE® group (P < 0.0001). The median time to clinical progression was 19.8 months in the ADT alone group vs 32.7 months in the ADT plus TAXOTERE® group (P < 0.0001). The authors concluded that this is the first study to demonstrate survival benefit in patients with newly diagnosed metastatic prostate cancer. This survival benefit with Androgen Deprivation Therapy and TAXOTERE® is even more so, in patients with high volume disease and should be considered standard treatment for those patients who are fit to receive TAXOTERE® based therapy. Sweeney C, Chen Y, Carducci MA, et al. 2014 ASCO Annual Meeting; LBA2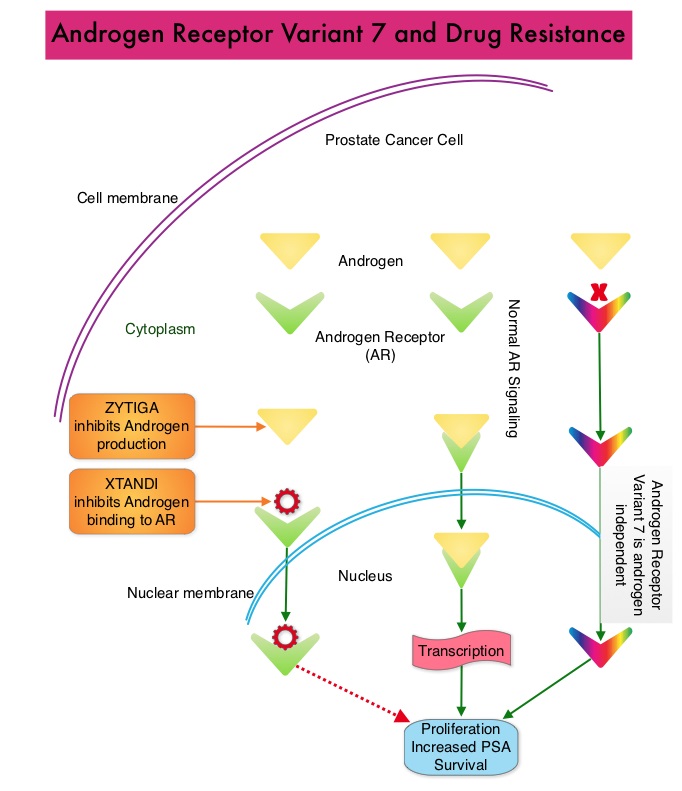 Upon progression {described as Castrate Resistant Prostate Cancer (CRPC), as these tumors are not androgen independent and continue to rely on Androgen Receptor signaling} two agents are presently available for metastatic CRPC. They include ZYTIGA® (Abiraterone) and XTANDI® (Enzalutamide). Both these agents have been shown to improve survival in metastatic CRPC. ZYTIGA® inhibits CYP 17A1 enzyme and depletes adrenal and intratumoral androgens, thereby impairing AR signaling. XTANDI® competes with Testosterone and Dihydrotestosterone and avidly binds to the Androgen Receptor, thereby inhibiting AR signaling and in addition inhibits translocation of the AR into the nucleus and thus inhibits the transcriptional activities of the AR. About 20-40% of the patients do not respond to these newer agents and even those who respond will invariably develop resistance to these drugs. This again has been attributed to persistent AR signaling by variant forms of Androgen Receptor, generated through somatic mutation or aberrant RNA splicing. Androgen Receptor Variant AR-V7 can be detected in the circulating tumor cells. AR-V7 does not have the domain to bind androgens and may be associated with resistance to XTANDI®. Further AR-V7 is constitutively active and can independently activate transcription factors and therefore is not effected by androgen depleting agents including ZYTIGA®. With this background, the authors hypothesized that detection of Androgen Receptor variant AR-V7 in circulating tumor cells from men with metastatic prostate cancer would be associated with resistance to both ZYTIGA® and XTANDI®. In this prospective study which enrolled patients with Castrate Resistant Prostate Cancer (CRPC), 31 patients received treatment with ZYTIGA® and 31 patients received treatment with XTANDI®. Levels of AR-V7 in circulating tumor cells of these patients were analyzed using quantitative Reverse Transcriptase – Polymerase Chain Reaction assay. The primary endpoint was association between AR-V7 status (positive versus negative) and Prostate Specific Antigen (PSA) response rates and secondary endpoints included freedom from PSA progression (PSA Progression Free Survival), clinical or radiographic Progression Free Survival, and Overall Survival. The authors noted that patients with detectable AR-V7 in circulating tumor cells had no response to ZYTIGA® or XTANDI® as measured by serum PSA level reduction of 50% or more and also had a shorter Progression Free Survival and Overall Survival. Also of interest, the prevalence of detectable AR-V7 in circulating tumor cells before treatment with ZYTIGA® and XTANDI® was 9-15% whereas it increased to approximately 50% after disease progressed during treatment with either of these two drugs. This suggested a common mechanism of resistance to both drugs. The authors concluded that detection of AR-V7 in circulating tumor cells from patients with Castration Resistant Prostate Cancer, may be associated with resistance to ZYTIGA® and XTANDI® and if further validated, could be used as a biomarker. Antonarakis ES, Lu C, Wang H, et al. N Engl J Med 2014; 371:1028-1038
Upon progression {described as Castrate Resistant Prostate Cancer (CRPC), as these tumors are not androgen independent and continue to rely on Androgen Receptor signaling} two agents are presently available for metastatic CRPC. They include ZYTIGA® (Abiraterone) and XTANDI® (Enzalutamide). Both these agents have been shown to improve survival in metastatic CRPC. ZYTIGA® inhibits CYP 17A1 enzyme and depletes adrenal and intratumoral androgens, thereby impairing AR signaling. XTANDI® competes with Testosterone and Dihydrotestosterone and avidly binds to the Androgen Receptor, thereby inhibiting AR signaling and in addition inhibits translocation of the AR into the nucleus and thus inhibits the transcriptional activities of the AR. About 20-40% of the patients do not respond to these newer agents and even those who respond will invariably develop resistance to these drugs. This again has been attributed to persistent AR signaling by variant forms of Androgen Receptor, generated through somatic mutation or aberrant RNA splicing. Androgen Receptor Variant AR-V7 can be detected in the circulating tumor cells. AR-V7 does not have the domain to bind androgens and may be associated with resistance to XTANDI®. Further AR-V7 is constitutively active and can independently activate transcription factors and therefore is not effected by androgen depleting agents including ZYTIGA®. With this background, the authors hypothesized that detection of Androgen Receptor variant AR-V7 in circulating tumor cells from men with metastatic prostate cancer would be associated with resistance to both ZYTIGA® and XTANDI®. In this prospective study which enrolled patients with Castrate Resistant Prostate Cancer (CRPC), 31 patients received treatment with ZYTIGA® and 31 patients received treatment with XTANDI®. Levels of AR-V7 in circulating tumor cells of these patients were analyzed using quantitative Reverse Transcriptase – Polymerase Chain Reaction assay. The primary endpoint was association between AR-V7 status (positive versus negative) and Prostate Specific Antigen (PSA) response rates and secondary endpoints included freedom from PSA progression (PSA Progression Free Survival), clinical or radiographic Progression Free Survival, and Overall Survival. The authors noted that patients with detectable AR-V7 in circulating tumor cells had no response to ZYTIGA® or XTANDI® as measured by serum PSA level reduction of 50% or more and also had a shorter Progression Free Survival and Overall Survival. Also of interest, the prevalence of detectable AR-V7 in circulating tumor cells before treatment with ZYTIGA® and XTANDI® was 9-15% whereas it increased to approximately 50% after disease progressed during treatment with either of these two drugs. This suggested a common mechanism of resistance to both drugs. The authors concluded that detection of AR-V7 in circulating tumor cells from patients with Castration Resistant Prostate Cancer, may be associated with resistance to ZYTIGA® and XTANDI® and if further validated, could be used as a biomarker. Antonarakis ES, Lu C, Wang H, et al. N Engl J Med 2014; 371:1028-1038
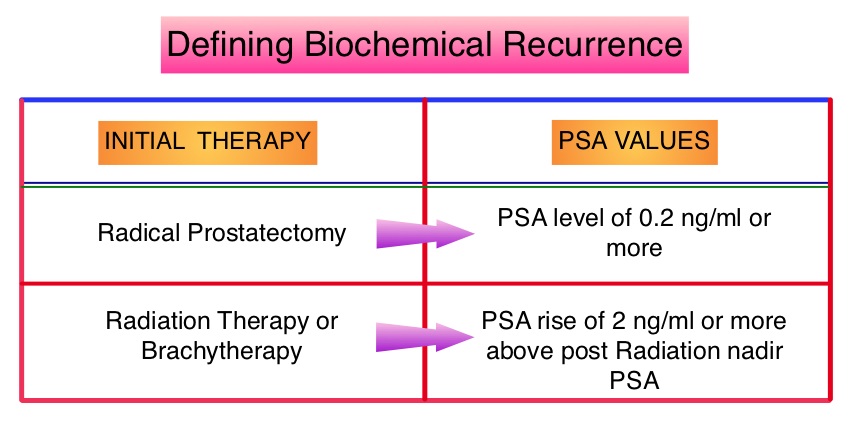 The American Urological Association suggested that a PSA of 0.2 ng/mL or higher defines PSA failure or relapse, after Radical Prostatectomy. A PSA rise of 2 ng/ml or more above post Radiation Therapy nadir, is considered PSA failure or relapse. Approximately 35% of the patients with prostate cancer will experience PSA only relapse within 10 years of their primary treatment and a third of these patients will develop documented metastatic disease within 8 years following PSA only relapse. Prostate cancer patients who had thought that they were cured, consequently can experience considerable mental anguish and anxiety, based on these laboratory findings. Androgen Deprivation Therapy (ADT) is often initiated following PSA only relapse with the intent of delaying disease progression although the role of ADT and optimal timing to start ADT (Immediate vs deferred ADT) in this patient population is unknown. Further, ADT can be associated with side effects such as fatigue, loss of muscle mass, impotence, anemia, osteoporosis, etc., which in turn can have a significant negative impact on an individual’s quality of life. In order to determine the significance of benefit if any, with starting ADT while patients are asymptomatic, the authors analyzed data on more than 14,000 patients included in a prospective registry called CaPSURE (Cancer of the Prostate Strategic Urologic Research Endeavor) and of them studied 2,022 men, who had experienced a PSA only relapse following curative surgery or radiation. These patients had clinical stage T3a,N0M0 or lower stage prostate cancer and experienced PSA only relapse (defined as PSA of 0.2 ng/mL or higher after Radical Prostatectomy or three rising PSA values one month apart following radiation treatment. Patients with symptoms, documented metastatic disease by CT scan or bone scan and ADT in the previous 12 months were excluded. Patients in the” Immediate treatment group” initiated ADT within 3 months of PSA relapse and those in the “Deferred treatment group” initiated ADT, 2 or more years after PSA relapse or when they presented with metastasis, symptoms or had a short PSA doubling time. The median age was 69 years, 34% had a Gleason score >7 and 32% received radiotherapy as primary treatment. The median time from primary treatment to PSA relapse was 27 months. Patients were followed for a median of 52.3 months after PSA relapse. The Five-year survival rate for Patients in the” Immediate treatment group” was 85.1% and for those in the “Deferred treatment group” was 87.2% with no significant difference in the all cause mortality. The 10 year survival was identical in both groups at 71.6%. The authors concluded that there is little or no survival benefit for Immediate ADT initiation compared with Deferred ADT initiation (at clinical progression or at least two years after PSA relapse) among prostate cancer patients with PSA only relapse. Therefore delaying ADT for at least 2 years after PSA relapse, following curative therapy for prostate cancer does not worsen overall survival. The findings from this large observational study will need further validation and a randomized phase III trial is underway to confirm these findings. Garcia-Albeniz X, Chan JM, Paciorek AT, et al. J Clin Oncol 32:5s, 2014 (suppl; abstr 5003)
The American Urological Association suggested that a PSA of 0.2 ng/mL or higher defines PSA failure or relapse, after Radical Prostatectomy. A PSA rise of 2 ng/ml or more above post Radiation Therapy nadir, is considered PSA failure or relapse. Approximately 35% of the patients with prostate cancer will experience PSA only relapse within 10 years of their primary treatment and a third of these patients will develop documented metastatic disease within 8 years following PSA only relapse. Prostate cancer patients who had thought that they were cured, consequently can experience considerable mental anguish and anxiety, based on these laboratory findings. Androgen Deprivation Therapy (ADT) is often initiated following PSA only relapse with the intent of delaying disease progression although the role of ADT and optimal timing to start ADT (Immediate vs deferred ADT) in this patient population is unknown. Further, ADT can be associated with side effects such as fatigue, loss of muscle mass, impotence, anemia, osteoporosis, etc., which in turn can have a significant negative impact on an individual’s quality of life. In order to determine the significance of benefit if any, with starting ADT while patients are asymptomatic, the authors analyzed data on more than 14,000 patients included in a prospective registry called CaPSURE (Cancer of the Prostate Strategic Urologic Research Endeavor) and of them studied 2,022 men, who had experienced a PSA only relapse following curative surgery or radiation. These patients had clinical stage T3a,N0M0 or lower stage prostate cancer and experienced PSA only relapse (defined as PSA of 0.2 ng/mL or higher after Radical Prostatectomy or three rising PSA values one month apart following radiation treatment. Patients with symptoms, documented metastatic disease by CT scan or bone scan and ADT in the previous 12 months were excluded. Patients in the” Immediate treatment group” initiated ADT within 3 months of PSA relapse and those in the “Deferred treatment group” initiated ADT, 2 or more years after PSA relapse or when they presented with metastasis, symptoms or had a short PSA doubling time. The median age was 69 years, 34% had a Gleason score >7 and 32% received radiotherapy as primary treatment. The median time from primary treatment to PSA relapse was 27 months. Patients were followed for a median of 52.3 months after PSA relapse. The Five-year survival rate for Patients in the” Immediate treatment group” was 85.1% and for those in the “Deferred treatment group” was 87.2% with no significant difference in the all cause mortality. The 10 year survival was identical in both groups at 71.6%. The authors concluded that there is little or no survival benefit for Immediate ADT initiation compared with Deferred ADT initiation (at clinical progression or at least two years after PSA relapse) among prostate cancer patients with PSA only relapse. Therefore delaying ADT for at least 2 years after PSA relapse, following curative therapy for prostate cancer does not worsen overall survival. The findings from this large observational study will need further validation and a randomized phase III trial is underway to confirm these findings. Garcia-Albeniz X, Chan JM, Paciorek AT, et al. J Clin Oncol 32:5s, 2014 (suppl; abstr 5003) Based on the recent research, it appears that there is an indirect link between mutations of the Androgen Receptor gene and the initiation and promotion of prostate cancer. On the contrary, low testosterone levels prior to therapy may be an independent predictor of a more aggressive disease, with an increased likelihood of extra-prostatic disease at the time of diagnosis and unfavorable treatment response. The current recommendations are to exclude prostate cancer before initiating testosterone replacement therapy in hypogonadal men over age 40, with a digital rectal examination (DRE) and PSA level and to closely monitor in the first year of testosterone replacement with DRE and PSA evaluations every 3 months and then semiannually. These recommendations are arbitrary, and not supported by published data. To determine the incidence/risk of prostate cancer with testosterone replacement therapy, the authors in this study reviewed the outcomes of 942 men in three cohorts, with testosterone levels less than or equal to 12.1 nmol/L from three German centers, who had received testosterone undecanoate for up to 16 years. The incidence of prostate cancer in cohort A (N=300) was 39.4 per 10,000 person-years (39.4 cases per 10,000 persons followed for 1 year), in cohort B (N=261) was 54.5 per 10,000 person-years (54.5 cases per 10,000 persons followed for 1 year) and in cohort C (N=381), no person was diagnosed with prostate cancer. The authors pointed out that the incidence of prostate cancer in the Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial was 116 per 10,000 person-years. Even though this review of registry data cannot be directly compared with screening trials, the authors concluded that based on their registry studies, long-term testosterone treatment in hypogonadal men does not appear to increase prostate cancer risk. A recently published article, in the Annals of Pharmacotherapy by Baillargeon, et al. concluded that there was no increased risk of myocardial infarction when hypogonadal patients over age 65, were treated with intramuscular testosterone. As the debate continues on the risk/ benefits of testosterone replacement therapy, the heightened awareness will probably bring about more responsible prescribing of testosterone supplements. Haider A, Zitzmann M, Yassin A. J Clin Oncol 32, 2014 (suppl 4; abstr 119)
Based on the recent research, it appears that there is an indirect link between mutations of the Androgen Receptor gene and the initiation and promotion of prostate cancer. On the contrary, low testosterone levels prior to therapy may be an independent predictor of a more aggressive disease, with an increased likelihood of extra-prostatic disease at the time of diagnosis and unfavorable treatment response. The current recommendations are to exclude prostate cancer before initiating testosterone replacement therapy in hypogonadal men over age 40, with a digital rectal examination (DRE) and PSA level and to closely monitor in the first year of testosterone replacement with DRE and PSA evaluations every 3 months and then semiannually. These recommendations are arbitrary, and not supported by published data. To determine the incidence/risk of prostate cancer with testosterone replacement therapy, the authors in this study reviewed the outcomes of 942 men in three cohorts, with testosterone levels less than or equal to 12.1 nmol/L from three German centers, who had received testosterone undecanoate for up to 16 years. The incidence of prostate cancer in cohort A (N=300) was 39.4 per 10,000 person-years (39.4 cases per 10,000 persons followed for 1 year), in cohort B (N=261) was 54.5 per 10,000 person-years (54.5 cases per 10,000 persons followed for 1 year) and in cohort C (N=381), no person was diagnosed with prostate cancer. The authors pointed out that the incidence of prostate cancer in the Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial was 116 per 10,000 person-years. Even though this review of registry data cannot be directly compared with screening trials, the authors concluded that based on their registry studies, long-term testosterone treatment in hypogonadal men does not appear to increase prostate cancer risk. A recently published article, in the Annals of Pharmacotherapy by Baillargeon, et al. concluded that there was no increased risk of myocardial infarction when hypogonadal patients over age 65, were treated with intramuscular testosterone. As the debate continues on the risk/ benefits of testosterone replacement therapy, the heightened awareness will probably bring about more responsible prescribing of testosterone supplements. Haider A, Zitzmann M, Yassin A. J Clin Oncol 32, 2014 (suppl 4; abstr 119) Radium Ra 223 dichloride (XOFIGO®) is a bone seeking alpha particle emitter and by virtue of its chemical similarity to calcium is preferentially taken up by the bone and forms complexes with bone mineral, hydroxyapatite, in areas where there is increased bone turnover, such as bone metastases. XOFIGO® induces double stranded DNA breaks resulting in antitumor effects and has a very short range in tissues (around 2 and 10 cells), quickly losing energy, compared to beta or gamma radiation. The end result is less damage to the adjacent healthy tissues. Further, unlike Ra-226 which was first isolated by Madame Curie, XOFIGO® has a short half life of 11.4 days and rapidly decays, preventing significant radiation exposure. The ALSYMPCA (ALpharadin in SYMptomatic Prostate CAncer patients) study is a randomized, double-blind, phase III trial, in which 921 patients with Castrate Resistant Prostate Cancer (CRPC) with 2 or more symptomatic bone metastases and no known visceral metastases, were randomly assigned in a 2:1 ratio to receive either XOFIGO® along with best supportive care (N=614) or placebo with best supportive care (N=307). Enrolled patients either progressed on or had not received TAXOTERE® (Docetaxel) for a variety of reasons and 42% of the enrolled patients were chemotherapy naïve.
Radium Ra 223 dichloride (XOFIGO®) is a bone seeking alpha particle emitter and by virtue of its chemical similarity to calcium is preferentially taken up by the bone and forms complexes with bone mineral, hydroxyapatite, in areas where there is increased bone turnover, such as bone metastases. XOFIGO® induces double stranded DNA breaks resulting in antitumor effects and has a very short range in tissues (around 2 and 10 cells), quickly losing energy, compared to beta or gamma radiation. The end result is less damage to the adjacent healthy tissues. Further, unlike Ra-226 which was first isolated by Madame Curie, XOFIGO® has a short half life of 11.4 days and rapidly decays, preventing significant radiation exposure. The ALSYMPCA (ALpharadin in SYMptomatic Prostate CAncer patients) study is a randomized, double-blind, phase III trial, in which 921 patients with Castrate Resistant Prostate Cancer (CRPC) with 2 or more symptomatic bone metastases and no known visceral metastases, were randomly assigned in a 2:1 ratio to receive either XOFIGO® along with best supportive care (N=614) or placebo with best supportive care (N=307). Enrolled patients either progressed on or had not received TAXOTERE® (Docetaxel) for a variety of reasons and 42% of the enrolled patients were chemotherapy naïve. The primary endpoint was Overall Survival and secondary endpoints included time to first symptomatic skeletal event, time to increase in total alkaline phosphatase level and PSA level. This study was stopped earlier than planned, as there was a significant increase in the median overall survival in the XOFIGO® group compared to placebo group, with a 30% reduction in the risk of death (14.9 months vs 11.3 months, HR=0.70, P<0.001). All secondary endpoints favored XOFIGO® as well. The most common adverse events were cytopenias, majority of which were Grades 1 and 2 and discontinuation rates due to adverse events were lower in the XOFIGO® group (17%) compared to 21% for the Placebo group. At 1.5 years, the incidence of myelosuppression was 3%, there were no grade 3 or 4 non-hematologic toxicities and no reports of secondary malignancies in the XOFIGO® group. The authors concluded that based on the long term safety data, XOFIGO® has an excellent side-effect profile in patients with CRPC. Unlike the bone seeking beta emitters such as Strontium-89 and Samarium-153, XOFIGO®, an alpha emitter, is the only agent that has been shown to improve overall survival. Studies are underway evaluating the efficacy of chemotherapy in combination with XOFIGO®, in patients with CRPC and associated bone metastases. Nilsson S, Vogelzang NJ, Sartor AO, et al. J Clin Oncol 32, 2014 (suppl 4; abstr 9)
The primary endpoint was Overall Survival and secondary endpoints included time to first symptomatic skeletal event, time to increase in total alkaline phosphatase level and PSA level. This study was stopped earlier than planned, as there was a significant increase in the median overall survival in the XOFIGO® group compared to placebo group, with a 30% reduction in the risk of death (14.9 months vs 11.3 months, HR=0.70, P<0.001). All secondary endpoints favored XOFIGO® as well. The most common adverse events were cytopenias, majority of which were Grades 1 and 2 and discontinuation rates due to adverse events were lower in the XOFIGO® group (17%) compared to 21% for the Placebo group. At 1.5 years, the incidence of myelosuppression was 3%, there were no grade 3 or 4 non-hematologic toxicities and no reports of secondary malignancies in the XOFIGO® group. The authors concluded that based on the long term safety data, XOFIGO® has an excellent side-effect profile in patients with CRPC. Unlike the bone seeking beta emitters such as Strontium-89 and Samarium-153, XOFIGO®, an alpha emitter, is the only agent that has been shown to improve overall survival. Studies are underway evaluating the efficacy of chemotherapy in combination with XOFIGO®, in patients with CRPC and associated bone metastases. Nilsson S, Vogelzang NJ, Sartor AO, et al. J Clin Oncol 32, 2014 (suppl 4; abstr 9)