SUMMARY: Prostate Cancer is the most common cancer in American men and approximately 233,000 new cases will be diagnosed in 2014 and close to 30,000 men will die of the disease. The primary systemic intervention for patients with advanced prostate cancer is Androgen Deprivation Therapy (ADT). This can be accomplished by either surgical castration (bilateral orchicetomy) or medical castration, using LHRH (GnRH- Gonadotropin-Releasing Hormone) agonists. Majority of these patients will eventually develop progressive disease (Castrate Resistant Prostate cancer – CRPC), due to enhanced autocrine and /or paracrine synthesis of androgens or androgen precursors in the tumor micro environment. This has lead to the development of novel compounds that decrease androgen synthesis as well as androgen signaling in patients with CRPC.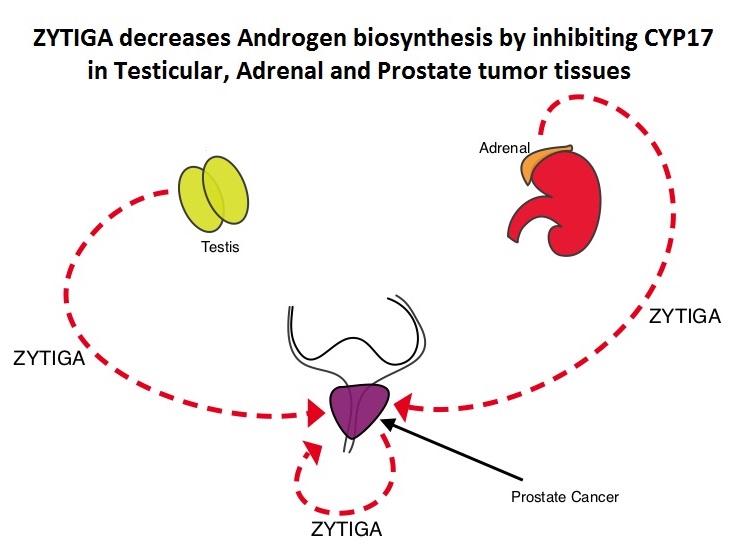 Abiraterone acetate (ZYTIGA®) is a novel, targeted, oral androgen biosynthesis inhibitor that decreases androgen production in the adrenal glands, testes and prostate cancer cells by inhibiting a steroidal enzyme CYP17A. Phase III trials have demonstrated that treatment with ZYTIGA® prolongs overall survival in metastatic CRPC patients, who had progressed after TAXOTERE® (Docetaxel) therapy, as well as those who are chemotherapy naive. ZYTIGA® delays deterioration of performance status, progression of fatigue and pain as well as development of skeletal related events, in TAXOTERE® refractory patients. It is important that any treatment considered for patients with asymptomatic or mildly symptomatic CRPC improves overall survival without negatively impacting Quality of Life. To address this further, the authors analyzed patient reported data related to pain and Quality of Life from a large randomized clinical trial. Of the 1088 chemotherapy-naïve, asymptomatic or mildly symptomatic CRPC patients randomized in this double-blind study, 546 patients received ZYTIGA® 1000 mg PO plus prednisone 5 mg twice daily and 542 patients received placebo plus prednisone. At the time of the planned interim analysis, ZYTIGA® improved radiographic progression-free survival, overall survival, and significantly delayed the initiation of chemotherapy. The authors in this publication reported the data related to pain and Quality of Life of these patients, at the time of the second preplanned interim analysis. Pain was assessed with the Brief Pain Inventory-Short Form (BPI-SF) questionnaire, which is a validated instrument to assess pain and Health Related Quality of Life (HRQoL) was measured with the Functional Assessment of Cancer Therapy—Prostate (FACT-P) questionnaire, which is a validated tool for metastatic CRPC. At a median follow-up of 22.2 months, the median time to progression of pain intensity was longer in patients receiving ZYTIGA® plus prednisone vs placebo plus prednisone (26.7 months vs 18.4 months, HR=0.82, P=0.049). The median time for pain to progress and interfere with daily activities was 10.3 months for ZYTIGA® vs 7.4 months for placebo (HR= 0.79, P=0.005). The median time to deterioration of HRQoL was longer in patients receiving ZYTIGA® plus prednisone vs those receiving placebo plus prednisone, as assessed by the FACT-P total score (12.7 months vs 8.3 months, HR=0.78, P=0.003). The authors concluded that ZYTIGA® given along with prednisone delays patient-reported pain progression and deterioration of HRQol, in chemotherapy-naive patients with metastatic CRPC, without compromising efficacy. Basch E, Autio K, Ryan CJ, et al. The Lancet Oncology 2013;14:1193 -1199
Abiraterone acetate (ZYTIGA®) is a novel, targeted, oral androgen biosynthesis inhibitor that decreases androgen production in the adrenal glands, testes and prostate cancer cells by inhibiting a steroidal enzyme CYP17A. Phase III trials have demonstrated that treatment with ZYTIGA® prolongs overall survival in metastatic CRPC patients, who had progressed after TAXOTERE® (Docetaxel) therapy, as well as those who are chemotherapy naive. ZYTIGA® delays deterioration of performance status, progression of fatigue and pain as well as development of skeletal related events, in TAXOTERE® refractory patients. It is important that any treatment considered for patients with asymptomatic or mildly symptomatic CRPC improves overall survival without negatively impacting Quality of Life. To address this further, the authors analyzed patient reported data related to pain and Quality of Life from a large randomized clinical trial. Of the 1088 chemotherapy-naïve, asymptomatic or mildly symptomatic CRPC patients randomized in this double-blind study, 546 patients received ZYTIGA® 1000 mg PO plus prednisone 5 mg twice daily and 542 patients received placebo plus prednisone. At the time of the planned interim analysis, ZYTIGA® improved radiographic progression-free survival, overall survival, and significantly delayed the initiation of chemotherapy. The authors in this publication reported the data related to pain and Quality of Life of these patients, at the time of the second preplanned interim analysis. Pain was assessed with the Brief Pain Inventory-Short Form (BPI-SF) questionnaire, which is a validated instrument to assess pain and Health Related Quality of Life (HRQoL) was measured with the Functional Assessment of Cancer Therapy—Prostate (FACT-P) questionnaire, which is a validated tool for metastatic CRPC. At a median follow-up of 22.2 months, the median time to progression of pain intensity was longer in patients receiving ZYTIGA® plus prednisone vs placebo plus prednisone (26.7 months vs 18.4 months, HR=0.82, P=0.049). The median time for pain to progress and interfere with daily activities was 10.3 months for ZYTIGA® vs 7.4 months for placebo (HR= 0.79, P=0.005). The median time to deterioration of HRQoL was longer in patients receiving ZYTIGA® plus prednisone vs those receiving placebo plus prednisone, as assessed by the FACT-P total score (12.7 months vs 8.3 months, HR=0.78, P=0.003). The authors concluded that ZYTIGA® given along with prednisone delays patient-reported pain progression and deterioration of HRQol, in chemotherapy-naive patients with metastatic CRPC, without compromising efficacy. Basch E, Autio K, Ryan CJ, et al. The Lancet Oncology 2013;14:1193 -1199
Tag: Prostate Cancer
Baseline Selenium Status and Effects of Selenium and Vitamin E Supplementation on Prostate Cancer Risk
SUMMARY: Selenium and Vitamin E Cancer Prevention Trial (SELECT), is a multicenter, randomized, placebo-controlled trial, conducted by the SWOG cooperative group, that involved more than 35,000 men. Participants were randomized to receive either, a) Selenium and Vitamin E, b) Selenium and a placebo, c) Vitamin E and a placebo or d) Two placebos. The purpose of this trial was to determine if high dose vitamin E (400 IU/day) and/or Selenium (200 mcg/day) supplements could decrease the incidence of prostate cancer. The level/concentration of Selenium in participants toenail clippings was measured at the time of study participation and the goal was to also determine whether Selenium supplements would benefit the subset of participants with low Selenium levels at baseline. Both Vitamin E and Selenium are antioxidants and Vitamin E rich foods include vegetables, vegetable oils, nuts, and egg yolks whereas Selenium a nonmetallic trace element is found in rice, wheat, seafood, meat, and Brazil nuts. 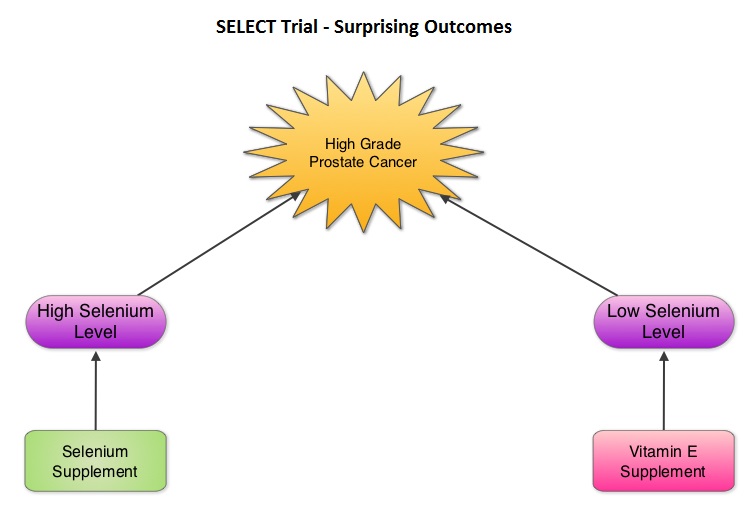 The SELECT trial, which began in 2001, was stopped early in 2008, as Selenium and Vitamin E, taken alone or together for an average of five and a half years did not decrease the incidence of prostate cancer. In 2011, an update on the SELECT trial data suggested that men who were randomized to the vitamin E alone had a 17 percent increased risk of prostate cancer compared to those men taking placebo. The authors in this case–cohort study continued follow up of the SELECT trial participants and with the Selenium levels data from toenail clippings, compared the effect of Selenium and Vitamin E, taken either alone or together, on the risk of prostate cancer, among 1739 men who were diagnosed with prostate cancer, of whom 489 participants developed high-grade prostate cancer. The control group for comparison was a random sample of 3117 men without prostate cancer and they were matched to the cases by race and age. It was noted that an individual’s baseline Selenium level, in the absence of supplementation, was not associated with prostate cancer risk. However, in men who had high baseline Selenium levels, Selenium supplements almost doubled (91%) the risk of high grade prostate cancer (P=0.007). Conversely, Vitamin E supplements had no effect among men with high baseline Selenium levels but doubled the risk of high grade prostate cancer among men with low baseline Selenium levels. Frankel et al. in an accompanying editorial point out that the dose of Vitamin E in the SELECT trial was significantly higher (400 IU/day) than the dose that was selected in the Alpha-Tocopherol Beta Carotene (ATBC) Cancer Prevention trial (50 IU/day), a study that was designed to test Vitamin E and beta carotene for lung cancer prevention in smokers. In the ATBC trial, a decrease in the incidence of prostate cancer incidence was observed, although this was a secondary finding and this study was not designed to determine prostate cancer risk. They comment that high doses of Vitamin E (Alpha-Tocopherol), suppresses the more potentially beneficial serum Gamma-Tocopherol which is the prevalent dietary form of Vitamin E in the United States. Selenium deficiency in the U.S. is not common and any benefit with Selenium supplements can only be seen in those who are Selenium deficient and high doses may be detrimental. The authors concluded that in the SELECT trial, the combination of both Vitamin E and Selenium did not reduce the risk of prostate cancer or any other cancer or heart disease and was in fact harmful for a significant number of individuals. Therefore, men 55 years of age or more should avoid Vitamin E or Selenium supplements at doses that exceed the recommended dietary intake. Kristal AR, Darke AK, Morris JS, et al. J Natl Cancer Inst; First published online 22 February 2014, doi: 10.1093/jnci/djt456
The SELECT trial, which began in 2001, was stopped early in 2008, as Selenium and Vitamin E, taken alone or together for an average of five and a half years did not decrease the incidence of prostate cancer. In 2011, an update on the SELECT trial data suggested that men who were randomized to the vitamin E alone had a 17 percent increased risk of prostate cancer compared to those men taking placebo. The authors in this case–cohort study continued follow up of the SELECT trial participants and with the Selenium levels data from toenail clippings, compared the effect of Selenium and Vitamin E, taken either alone or together, on the risk of prostate cancer, among 1739 men who were diagnosed with prostate cancer, of whom 489 participants developed high-grade prostate cancer. The control group for comparison was a random sample of 3117 men without prostate cancer and they were matched to the cases by race and age. It was noted that an individual’s baseline Selenium level, in the absence of supplementation, was not associated with prostate cancer risk. However, in men who had high baseline Selenium levels, Selenium supplements almost doubled (91%) the risk of high grade prostate cancer (P=0.007). Conversely, Vitamin E supplements had no effect among men with high baseline Selenium levels but doubled the risk of high grade prostate cancer among men with low baseline Selenium levels. Frankel et al. in an accompanying editorial point out that the dose of Vitamin E in the SELECT trial was significantly higher (400 IU/day) than the dose that was selected in the Alpha-Tocopherol Beta Carotene (ATBC) Cancer Prevention trial (50 IU/day), a study that was designed to test Vitamin E and beta carotene for lung cancer prevention in smokers. In the ATBC trial, a decrease in the incidence of prostate cancer incidence was observed, although this was a secondary finding and this study was not designed to determine prostate cancer risk. They comment that high doses of Vitamin E (Alpha-Tocopherol), suppresses the more potentially beneficial serum Gamma-Tocopherol which is the prevalent dietary form of Vitamin E in the United States. Selenium deficiency in the U.S. is not common and any benefit with Selenium supplements can only be seen in those who are Selenium deficient and high doses may be detrimental. The authors concluded that in the SELECT trial, the combination of both Vitamin E and Selenium did not reduce the risk of prostate cancer or any other cancer or heart disease and was in fact harmful for a significant number of individuals. Therefore, men 55 years of age or more should avoid Vitamin E or Selenium supplements at doses that exceed the recommended dietary intake. Kristal AR, Darke AK, Morris JS, et al. J Natl Cancer Inst; First published online 22 February 2014, doi: 10.1093/jnci/djt456
Enzalutamide in men with chemotherapy-naive metastatic prostate cancer (mCRPC) Results of phase III PREVAIL study
SUMMARY: Prostate Cancer is driven by androgens (primarily testosterone) and androgen signaling pathways. There is evidence to suggest that prostate cancer cells continue to depend on androgen receptor (AR) signaling even in an androgen-deprived environment. Therefore, targeting AR and AR signaling pathways remains a rational approach in the treatment of Castration Resistant Prostate Cancer (CRPC). The first generation anti-androgen agents such as EULEXIN® (Flutamide), CASODEX® (Bicalutamide) and NILANDRON® (Nilutamide) act by binding to the Androgen Receptor (AR) and prevent the activation of the AR and subsequent up-regulation of androgen responsive genes. They may also accelerate the degradation of the AR. These agents have a range of pharmacologic activity from being pure anti-androgens to androgen agonists.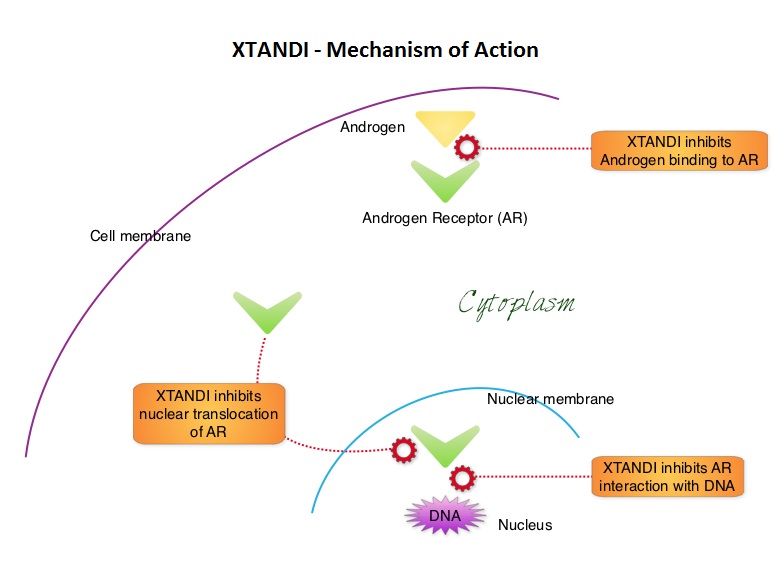 XTANDI® (Enzalutamide) is a second-generation anti-androgen with no reported agonistic effects. It competitively inhibits androgens and AR binding to androgens as well as AR nuclear translocation and interaction with DNA. It thus inhibits several steps in the AR signaling pathway. XTANDI® was first approved by the FDA in 2012, for the treatment of patients with metastatic CRPC who have previously received TAXOTERE® (Docetaxel) based chemotherapy. The PREVAIL study is a double-blind, placebo-controlled, phase III trial in which 1,717 chemotherapy-naive patients with mCRPC (metastatic Castrate Resistant Prostate Cancer) were randomly assigned 1:1 to receive either XTANDI® 160 mg/day or placebo. Prior treatment with surgery or radiation therapy for their primary tumor, as well as hormonal intervention with a LHRH (Luteinizing Hormone Releasing Hormone) agonist or first-generation anti-androgen was allowed. The two co-primary endpoints were Overall Survival (OS) and radiographic Progression Free Survival (rPFS), as measured by bone scans and CT scans. At the time of preplanned interim analysis, XTANDI® demonstrated a statistically significant benefit over placebo with a 30% reduction in risk of death (OS: HR= 0.70; P< 0.0001) and an 81% reduction in risk of radiographic Progression Free Survival (rPFS: HR 0.19; P< 0.0001). Further, the response rates were meaningful with 20% complete responses and 39% partial responses (59% Response Rate) compared with 5% Response Rate in the placebo group (P<0.0001). XTANDI® also significantly delayed the median time to chemotherapy by 17 months compared with those who took placebo (P<0.0001). Based on the results of this interim analysis, the Independent Data Monitoring Committee recommended stopping the study and allowing patients in the placebo group to receive XTANDI®. XTANDI® was well tolerated and the most common side effects were hot flashes, weight gain, fatigue, constipation, back and joint pain. The authors concluded that XTANDI® significantly improves OS and rPFS in patients with chemotherapy-naive mCRPC and can significantly delay the need for chemotherapeutic intervention. Beer TM, Armstrong AJ, Sternberg CN, et al. J Clin Oncol 32, 2014 (suppl 4; abstr LBA1)
XTANDI® (Enzalutamide) is a second-generation anti-androgen with no reported agonistic effects. It competitively inhibits androgens and AR binding to androgens as well as AR nuclear translocation and interaction with DNA. It thus inhibits several steps in the AR signaling pathway. XTANDI® was first approved by the FDA in 2012, for the treatment of patients with metastatic CRPC who have previously received TAXOTERE® (Docetaxel) based chemotherapy. The PREVAIL study is a double-blind, placebo-controlled, phase III trial in which 1,717 chemotherapy-naive patients with mCRPC (metastatic Castrate Resistant Prostate Cancer) were randomly assigned 1:1 to receive either XTANDI® 160 mg/day or placebo. Prior treatment with surgery or radiation therapy for their primary tumor, as well as hormonal intervention with a LHRH (Luteinizing Hormone Releasing Hormone) agonist or first-generation anti-androgen was allowed. The two co-primary endpoints were Overall Survival (OS) and radiographic Progression Free Survival (rPFS), as measured by bone scans and CT scans. At the time of preplanned interim analysis, XTANDI® demonstrated a statistically significant benefit over placebo with a 30% reduction in risk of death (OS: HR= 0.70; P< 0.0001) and an 81% reduction in risk of radiographic Progression Free Survival (rPFS: HR 0.19; P< 0.0001). Further, the response rates were meaningful with 20% complete responses and 39% partial responses (59% Response Rate) compared with 5% Response Rate in the placebo group (P<0.0001). XTANDI® also significantly delayed the median time to chemotherapy by 17 months compared with those who took placebo (P<0.0001). Based on the results of this interim analysis, the Independent Data Monitoring Committee recommended stopping the study and allowing patients in the placebo group to receive XTANDI®. XTANDI® was well tolerated and the most common side effects were hot flashes, weight gain, fatigue, constipation, back and joint pain. The authors concluded that XTANDI® significantly improves OS and rPFS in patients with chemotherapy-naive mCRPC and can significantly delay the need for chemotherapeutic intervention. Beer TM, Armstrong AJ, Sternberg CN, et al. J Clin Oncol 32, 2014 (suppl 4; abstr LBA1)
Long-Term Survival of Participants in the Prostate Cancer Prevention Trial
SUMMARY: In the landmark Prostate Cancer Prevention Trial (PCPT), Finasteride (PROSCAR®) reduced the risk of Prostate Cancer development and therefore the symptoms associated with it by 33%, compared to Placebo. However, in those who did develop Prostate Cancer while on PROSCAR®, there was an increased risk of high grade tumors. After 18 years of follow up of these patients, it appears that in spite of this set back, there was no difference in the overall survival between the PROSCAR® group and the placebo group. The U.S. Preventive Services Task Force (USPSTF) has been against Prostate Cancer screening, as the consensus is that majority of the Prostate Cancers detected by screening would never become apparent in an individual’s lifetime, if this individual was not screened and therefore would never cause a problem. This long term data begs a very important question – Is it worthwhile taking PROSCAR® for Prostate Cancer prevention? Thompson IM, Goodman PJ, Tangen CM, et al. N Engl J Med 2013;369:603-610
Prostate Cancer – Is Prevention Worthwhile?
In the landmark Prostate Cancer Prevention Trial (PCPT), Finasteride (PROSCAR®) reduced the risk of Prostate Cancer development and therefore the symptoms associated with it by 33%, compared to placebo. However, in those who did develop Prostate Cancer while on PROSCAR®, there was an increased risk of more aggressive disease. After 18 years of follow up of these patients (NEJM 2013), it appears that in spite of this set back, there was no difference in the overall survival between the PROSCAR® group and the placebo group. The U.S. Preventive Services Task Force (USPSTF) has been against Prostate Cancer screening, as the consensus is that majority of the Prostate Cancers detected by screening would never become apparent in an individual’s lifetime, if this individual was not screened and therefore would never cause a problem. This long term data begs a very important question – Is it worthwhile taking PROSCAR® for Prostate Cancer prevention?
Alpha Emitter Radium-223 and Survival in Metastatic Prostate Cancer
SUMMARY: Radium Ra 223 dichloride (XOFIGO®) is a bone seeking alpha emitter that selectively targets areas of increased bone turnover. It induces double–stranded DNA breaks and has a very limited range path and quickly loses energy within a short distance of its source. This results in less damage to the adjacent healthy tissue. Further, unlike the dreaded Ra 226 which was first isolated by Madame Curie, XOFIGO® has a short half life of 11.4 days and rapidly decays preventing radiation exposure. In a randomized, double-blind phase III trial, 921 patients with Castrate Resistant Prostate Cancer (CRPC) who had progressed on or had not received TAXOTERE® (Docetaxel) for a variety of reasons, were randomly assigned in a 2:1 ratio to receive either XOFIGO®, with best supportive care or PLACEBO with best supportive care. Patients with visceral metastases were excluded. The primary endpoint was overall survival and secondary endpoints included time to first symptomatic skeletal event, time to increase in total alkaline phosphatase level and PSA level. There was a significant increase in the median overall survival in the XOFIGO® group compared to placebo group with a 30% reduction in the risk of death (14.9 months vs 11.3 months, HR=0.70, P<0.001). All secondary endpoints favored XOFIGO® as well. All adverse events were lower in the XOFIGO® group and myelosuppression was minimal. Unlike the bone seeking beta emitters, Strontium-89 and Samarium-153, XOFIGO®, an alpha emitter, is the only agent that has been shown to improve overall survival. Studies are underway evaluating the efficacy of chemotherapy in combination with XOFIGO®, in patients with CRPC with bone metastases. Parker C, Nilsson S, Heinrich D, et al. N Eng J Med 2013;369:213-23
XOFIGO® (Radium Ra 223 dichloride)
XOFIGO® (Radium Ra 223 dichloride): The FDA on May 15, 2013 approved XOFIGO® Injection for the treatment of castration-resistant prostate cancer patients with symptomatic bone metastases and no known visceral metastatic disease. XOFIGO® is an alpha-particle emitting radiotherapeutic drug. It mimics calcium and forms complexes with hydroxyapatite at sites of increased bone turnover, as seen at metastatic lesions in the bone. XOFIGO® is a product of Bayer HealthCare Pharmaceuticals Inc.
Proton Versus Intensity-Modulated Radiotherapy for Prostate Cancer Patterns of Care and Early Toxicit
SUMMARY: Proton Radiotherapy (PRT) unlike external beam radiotherapy uses energy from positively charged particles called protons and has the ability to precisely localize the radiation dose to the tumor site, minimizing collateral damage. In this retrospective analysis, data on 27,647 Medicare beneficiaries who received PRT or IMRT (Intensity-Modulated RadioTherapy) for prostate cancer, was reviewed, during 2008 and/or 2009. At 12 months post-treatment, there was no statistical difference in genitourinary and gastrointestinal toxicity for patients who had received PRT compared to those who were treated with IMRT. With PRT almost twice as expensive as IMRT, it remains to be seen if PRT will be reimbursed by third party payors. It is interesting to note that in this study, patients receiving PRT were relatively younger, healthier, and lived in more affluent areas than patients receiving IMRT. Yu JB, Soulos PR, Herrin J, et al. J. Natl. Cancer Inst. 2013;105:25-32
PROTON BEAM RADIATION THERAPY (PBRT) for Prostate Cancer
PBRT is a type of external beam radiation therapy in which a beam of Proton particles are used to irradiate cancer tissue. It has been claimed that the main advantage of proton therapy is its ability to more precisely localize the radiation dosage to the tumor tissue and thereby improve tumor control, without causing significant damage to the surrounding tissues. This theoretical consideration was however disproved in a study published in the Jan 2, 2013 issue of the JNCI. In this study, data was gathered following prostate cancer treatment of over 22,000 medicare beneficiaries, with either IMRT (Intensity Modulated Radiation Therapy) or PBRT. The authors noted that PBRT was not superior to IMRT with regards to efficacy or toxicity, after one year. PBRT was however twice as expensive as IMRT. It therefore remains to be seen if third party payors will warm up to PBRT, with the expansion of PBRT centers across the country.
ZYTIGA® (Abiraterone)
The FDA on December 10, 2012 approved an expanded indication for ZYTIGA® in combination with prednisone for the treatment of patients with metastatic castration-resistant prostate cancer. ZYTIGA® tablets are a product of Janssen Biotech, Inc.
