SUMMARY: The American Cancer Society estimates that in 2018, about 55,440 people will be diagnosed with pancreatic cancer and about 44,330 people will die of the disease. Pancreatic cancer is the fourth most common cause of cancer-related deaths in the United States and Western Europe. Unfortunately, unlike other malignancies, very little progress has been made and outcomes for patients with advanced pancreatic cancer, has been dismal. Diagnosis is often made late in the course of the disease, as patients are often asymptomatic and early tumors cannot be detected during routine physical examination. Further, precursors of pancreatic cancer evolve as microscopic lesions in the ducts and are often not visualized on imaging studies. Based on the National Cancer Institute Data Base, the 5 year observed survival rate for patients diagnosed with exocrine cancer of the Pancreas is 14% for those with Stage IA disease, and 1% for those with Stage IV disease. Early detection and cancer prevention is therefore critical.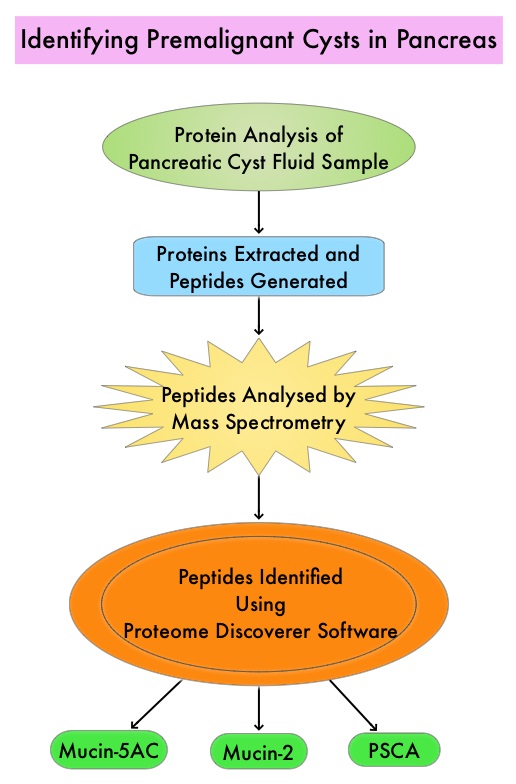
Pancreatic adenocarcinoma can also develop from mucin-producing Pancreatic Cystic Lesions (PCLs) and these neoplasms include Intraductal Papillary Mucinous Neoplasms (IPMNs) and Mucinous Cystic Neoplasms. These neoplasms comprise 10-50% of Pancreatic Cystic Lesions (PCLs). It should be noted that PCLs also encompass intrinsically benign tumors such as serous cystic neoplasms and inflammatory pseudocysts. With the rising use of abdominal MRI, partly due to concerns about ionizing radiation inherent to other exams such as CT scans, PCLs are incidentally discovered in up to 20% of these imaging studies in adults and these individuals are asymptomatic. Imaging techniques that are presently available cannot distinguish between benign, premalignant, and malignant PCLs. The same is true for currently available Endoscopic Ultrasound (EUS)-guided Fine Needle Aspiration (FNA) of Pancreatic Cystic Lesions and evaluation of cyst fluid for cytology and quantification of CarcinoEmbryonic Antigen (CEA). A high risk lesion in the pancreas would require surgical intervention with associated risks. Identifying benign from premalignant and malignant PCLs, as well as determining the epithelial subtype of IPMNs is therefore critical. The risk of malignancy is highest for Pancreatobiliary-type IPMNs with somewhat better prognosis for Intestinal-type IPMNs, whereas Gastric-type IPMNs tend to be indolent.
The authors in this study utilized targeted Mass Spectrometry (MS) to identify and quantitate proteins in the cystic fluid samples. Targeted quantitation of proteins by Mass Spectrometry provides a next-generation platform that overcomes many of the limitations of Western blotting and provides new capabilities for protein analysis. This sensitive technique is used to detect, identify and quantitate protein molecules in a given sample, based on their mass-to-charge ratio, enabling targeted protein measurement.
Using pancreatic cyst fluid samples obtained by routine EUS-guided FNA, biomarker candidates for malignant potential and high-grade dysplasia/cancer were identified via an explorative proteomic approach, in an initial cohort of 24 patients. Subsequently, a quantitative analysis using 30 heavy-labeled peptides from the biomarkers and parallel reaction monitoring mass spectrometry was devised, and tested, in a training cohort of 80 patients, and prospectively evaluated in a validation cohort of 68 patients. Patients with solid-pseudopapillary neoplasm and neuroendocrine tumor were excluded. The Primary objective of this study was to devise and validate a targeted, quantitative proteomic analysis to identify and distinguish between premalignant Pancreatic Cystic Lesions (PCLs) and Cystic neoplasms with manifest high-grade dysplasia /cancer. A Secondary aim was to find and evaluate markers for different epithelial subtypes of IPMNs, which may be used to predict the risk of malignant transformation.
It was noted that the optimal set of markers for detecting malignant potential was a panel of peptides from Mucin-5AC and Mucin-2, which could distinguish premalignant/malignant lesions from benign, with an accuracy of 97% in the validation cohort , compared with 61% using pancreatic cyst fluid CarcinoEmbryonic Antigen (P< 0.001) and 84% using Cytology (P=0.02). A combination of proteins Mucin-5AC and Prostate Stem Cell Antigen (PSCA) could identify high-grade dysplasia/cancer with an accuracy of 96% and detected 95% of malignant/severely dysplastic lesions, compared with 35% and 50% for CarcinoEmbryonic Antigen and Cytology (P<0.001 and P=0.003, respectively).
The authors concluded that Targeted Mass Spectrometry analysis of three pancreatic cyst fluid biomarkers provides highly accurate identification and assessment of cystic precursors to pancreatic adenocarcinoma. It remains to be seen whether this methodology will be beneficial for early diagnosis as well as prevention of development of pancreatic adenocarcinoma. Highly Accurate Identification of Cystic Precursor Lesions of Pancreatic Cancer Through Targeted Mass Spectrometry: A Phase IIc Diagnostic Study. Jabbar KS, Arike L, Hansson GC, et al. J Clin Oncol 2018;36:367-375

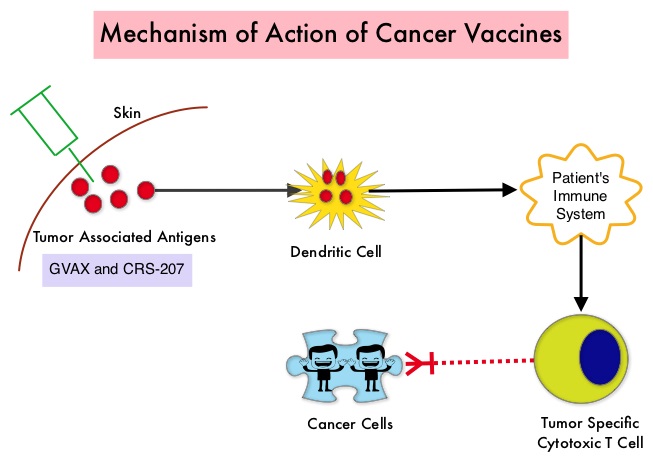 The two vaccines studied were GVAX and CRS-207. GVAX is an allogeneic whole cell vaccine developed from Pancreatic Cancer cell lines. These cancer cells are irradiated, to prevent them from dividing and are genetically modified to secrete GM-CSF (Granulocyte Macrophage Colony Stimulating Factor). GM-CSF is important for the growth and activation of dendritic cells also known as Antigen Presenting Cells. This vaccine when injected attracts the dendritic cells to the vaccine injection site and the dendritic cells in turn, pick up the antigens from the vaccine and present them to the patient’s immune system. The immune system then mounts a response by activating tumor specific T-cells. This vaccine therefore theoretically boosts the body’s immune system to fight the patient’s tumor, without causing collateral damage. The second vaccine CRS-207 is live-attenuated (weakened) Listeria monocytogenes bacterium which expresses mesothelin and stimulates innate and adaptive immunity. It is genetically engineered to elicit an immune response against the tumor-associated antigen mesothelin, which has been shown to be expressed at higher levels on Pancreatic Cancer cells than on normal cells. Previous studies have demonstrated that survival can be improved by induction of mesothelin specific T-cell responses. In this study, 90 patients with metastatic Pancreatic Adenocarcinoma were randomly assigned in a 2:1 ratio to receive two doses of GVAX followed by four doses of CRS-207 in Group A and six doses of GVAX alone in the Group B. Treatment was given every 3 weeks and low-dose CYTOXAN® (Cyclophosphamide) was given IV, the day before GVAX in both groups, to inhibit regulatory (suppressive) T-cells. More than 80% of the patients had at least one prior treatment for metastatic disease and 50% had two or more prior treatments. The Primary endpoint was overall survival. Secondary endpoints included safety, clinical and immune responses. At a planned interim analysis, the median Overall Survival (OS) was 6.1 months in Group A patients who had received the combination of two vaccines compared to 3.9 months in Group B patients who received GVAX alone (HR=0.59, P=0.02), resulting in a 41% reduction in risk of death with the combination immunotherapy. The one year survival probability doubled with the dual vaccine with an estimated one year survival of 24% for the combination immunotherapy group (Group A) compared with 12% for the GVAX alone group (Group B). The median OS in an updated analysis of patients who received three total doses which included at least two doses of GVAX and at least one dose of CRS-207 was 9.7 months compared to 4.6 months for those who received three doses of GVAX alone (HR=0.53, P=0.02), a 47% reduction in the risk of death. In the subgroup of patients who had two or more prior chemotherapy regimens, combination immunotherapy given as third line therapy or greater resulted in a median OS of 5.7 months compared to 3.7 months with GVAX alone (HR=0.30, P<0.001), a 70% reduction in risk of death. Stabilization or reduction of tumor marker CA 19-9, was seen in 27% of patients receiving combination immunotherapy compared to 9% in those who received GVAX alone (P=0.08). The median OS in patients with stable or better CA 19-9 response was 10.3 months compared with 4 months in those with CA 19-9 progression (HR=0.43, P=0.02). Toxicities included local reactions after GVAX and transient fevers, chills, and lymphopenia after CRS-207 administration. The authors concluded that immunotherapy with a combination of two vaccines improved Overall Survival with minimal toxicity, for patients with metastatic Pancreatic Carcinoma, who had failed prior therapies. Safety and Survival With GVAX Pancreas Prime and Listeria Monocytogenes–Expressing Mesothelin (CRS-207) Boost Vaccines for Metastatic Pancreatic Cancer. Le DT, Wang-Gillam A, Picozzi V, et al. J Clin Oncol 2015; 33:1325-1333
The two vaccines studied were GVAX and CRS-207. GVAX is an allogeneic whole cell vaccine developed from Pancreatic Cancer cell lines. These cancer cells are irradiated, to prevent them from dividing and are genetically modified to secrete GM-CSF (Granulocyte Macrophage Colony Stimulating Factor). GM-CSF is important for the growth and activation of dendritic cells also known as Antigen Presenting Cells. This vaccine when injected attracts the dendritic cells to the vaccine injection site and the dendritic cells in turn, pick up the antigens from the vaccine and present them to the patient’s immune system. The immune system then mounts a response by activating tumor specific T-cells. This vaccine therefore theoretically boosts the body’s immune system to fight the patient’s tumor, without causing collateral damage. The second vaccine CRS-207 is live-attenuated (weakened) Listeria monocytogenes bacterium which expresses mesothelin and stimulates innate and adaptive immunity. It is genetically engineered to elicit an immune response against the tumor-associated antigen mesothelin, which has been shown to be expressed at higher levels on Pancreatic Cancer cells than on normal cells. Previous studies have demonstrated that survival can be improved by induction of mesothelin specific T-cell responses. In this study, 90 patients with metastatic Pancreatic Adenocarcinoma were randomly assigned in a 2:1 ratio to receive two doses of GVAX followed by four doses of CRS-207 in Group A and six doses of GVAX alone in the Group B. Treatment was given every 3 weeks and low-dose CYTOXAN® (Cyclophosphamide) was given IV, the day before GVAX in both groups, to inhibit regulatory (suppressive) T-cells. More than 80% of the patients had at least one prior treatment for metastatic disease and 50% had two or more prior treatments. The Primary endpoint was overall survival. Secondary endpoints included safety, clinical and immune responses. At a planned interim analysis, the median Overall Survival (OS) was 6.1 months in Group A patients who had received the combination of two vaccines compared to 3.9 months in Group B patients who received GVAX alone (HR=0.59, P=0.02), resulting in a 41% reduction in risk of death with the combination immunotherapy. The one year survival probability doubled with the dual vaccine with an estimated one year survival of 24% for the combination immunotherapy group (Group A) compared with 12% for the GVAX alone group (Group B). The median OS in an updated analysis of patients who received three total doses which included at least two doses of GVAX and at least one dose of CRS-207 was 9.7 months compared to 4.6 months for those who received three doses of GVAX alone (HR=0.53, P=0.02), a 47% reduction in the risk of death. In the subgroup of patients who had two or more prior chemotherapy regimens, combination immunotherapy given as third line therapy or greater resulted in a median OS of 5.7 months compared to 3.7 months with GVAX alone (HR=0.30, P<0.001), a 70% reduction in risk of death. Stabilization or reduction of tumor marker CA 19-9, was seen in 27% of patients receiving combination immunotherapy compared to 9% in those who received GVAX alone (P=0.08). The median OS in patients with stable or better CA 19-9 response was 10.3 months compared with 4 months in those with CA 19-9 progression (HR=0.43, P=0.02). Toxicities included local reactions after GVAX and transient fevers, chills, and lymphopenia after CRS-207 administration. The authors concluded that immunotherapy with a combination of two vaccines improved Overall Survival with minimal toxicity, for patients with metastatic Pancreatic Carcinoma, who had failed prior therapies. Safety and Survival With GVAX Pancreas Prime and Listeria Monocytogenes–Expressing Mesothelin (CRS-207) Boost Vaccines for Metastatic Pancreatic Cancer. Le DT, Wang-Gillam A, Picozzi V, et al. J Clin Oncol 2015; 33:1325-1333