SUMMARY: The American Cancer Society estimates that in the United States for 2018, about 8,500 new cases of Hodgkin lymphoma will be diagnosed and about 1,050 patients will die of the disease. Hodgkin lymphoma is classified into two main groups – Classical Hodgkin lymphomas and Nodular Lymphocyte Predominant type, by the World Health Organization. The Classical Hodgkin lymphomas include Nodular sclerosing, Mixed cellularity, Lymphocyte rich, Lymphocyte depleted subtypes and accounts for approximately 10% of all malignant lymphomas. Nodular sclerosis Hodgkin lymphoma histology, accounts for approximately 80% of Hodgkin lymphoma cases in older children and adolescents in the United States. Classical Hodgkin Lymphoma is a malignancy of primarily B lymphocytes and is characterized by the presence of large mononucleated Hodgkin (H) and giant multinucleated Reed-Sternberg (RS) cells, collectively known as Hodgkin and Reed-Sternberg cells (HRS).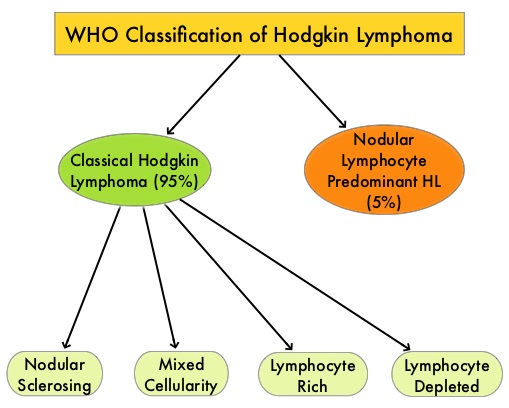
For patients with Hodgkin Lymphoma, the goal of first-line chemotherapy is cure. A positive PET scan following first-line chemotherapy is indicative of incomplete response with residual disease and warrants subsequent chemotherapy or radiation. Advanced stage (stage III to stage IV) Classical Hodgkin lymphoma has a cure rate of approximately 70-80% when treated in the first-line setting with a combination of Doxorubicin, Bleomycin, Vinblastine, and Dacarbazine (ABVD). This regimen which was developed more than 40 years ago is less expensive, easy to administer, is generally well tolerated and is often used in first line setting. Nonetheless, this regimen which contains Bleomycin can cause pulmonary toxicity, the incidence of which is higher in older patients and in those who receive consolidation radiotherapy to the thorax.
ADCETRIS® (Brentuximab vedotin) is an antibody-drug conjugate (ADC) that targets CD30, which is a surface antigen, expressed on Reed-Sternberg cells, in patients with Classical Hodgkin lymphoma. This ADC consists of an anti-CD30 monoclonal antibody linked to MonoMethyl Auristatin E (MMAE), an antimicrotubule agent. Upon binding to the CD30 molecule on the cancer cells, MMAE is released into the cancer cell, resulting in cell death. ADCETRIS® is presently approved by the FDA for the treatment of Classical Hodgkin lymphoma, after failure of Autologous Hematopoietic Stem Cell Transplantation (auto-HSCT) or after failure of at least two prior multi-agent chemotherapy regimens in patients who are not auto-HSCT candidates. It is also approved for Classical Hodgkin lymphoma at high risk of relapse or progression, as auto-HSCT consolidation.
In a previously published phase I study, ADCETRIS® in combination with AVD (A+AVD) resulted in a Complete Response rate of 96% and a 5 year Overall Survival rate of 100%. Based on these finding, ECHELON-1 study was conducted, which is an international, open-label, randomized, multicenter, phase III trial, comparing A+AVD with ABVD, as frontline therapy in patients with stage III or IV Classical Hodgkin lymphoma.
This study included 1334 previously untreated patients with stage III or IV Classical Hodgkin lymphoma, who were randomly assigned in a 1:1 ratio to receive A+AVD (N=664), which consisted of ADCETRIS® 1.2 mg/kg , Doxorubicin 25 mg/m2, Vinblastine 6 mg/m2 and Dacarbazine 375 mg/m2 or ABVD (N=670), which consisted of Doxorubicin 25 mg/m2, Bleomycin 10 units/m2, Vinblastine 6 mg/m2 and Dacarbazine 375 mg/m2, given intravenously, on days 1 and 15 of each 28-day cycle, for up to 6 cycles. The Primary end point was “modified” Progression Free Survival (mPFS), which, in addition to disease progression or death, included less than Complete Response after the completion of frontline chemotherapy, based on independently assessed PET results. PET scan interpretation was based on Deauville score (The Deauville score is a 5-point scale on which higher scores indicate greater uptake of FDG glucose at involved sites on PET). Patients were stratified according to International Prognostic Score (IPS) risk group (low risk vs. intermediate risk vs. high risk). A PET scan was performed at the end of the second cycle of treatment (PET2) and patients were offered alternative frontline therapy at the discretion of the treating physician, for patients with a PET Deauville score of 5. Secondary end points included Overall Survival.
At a median follow up of 24.6 months, the 2 year modified PFS in the A+AVD and ABVD groups were 82.1% and 77.2% respectively ( HR=0.77; P=0.04). All Secondary end points also trended in favor of A+AVD. Further, the benefit of A+AVD was noted across all prespecified subgroups, including those with involvement of more than one extranodal site, patients with a high IPS risk score and stage IV disease. Additionally, a higher proportion of the patients treated with A+AVD had negative PET2 results than those treated with ABVD (89% versus 86%). There was however a higher incidence of neutropenia in the A+AVD group, but this was alleviated with G-CSF prophylaxis. There was a higher incidence of peripheral neuropathy in the A+AVD group as well, and this improved or resolved over time. Pulmonary toxicity was lower in patients receiving A+AVD compared to those receiving ABVD.
The authors concluded that at 2 years, among patients with advanced stage Hodgkin lymphoma, A+AVD had superior efficacy when compared to ABVD, with a lower combined risk of progression, death or incomplete response and subsequent use of anticancer therapy. Brentuximab Vedotin with Chemotherapy for Stage III or IV Hodgkin’s Lymphoma. Connors JM, Jurczak W, Straus DJ, et al., for the ECHELON-1 Study Group. N Engl J Med 2018; 378:331-344


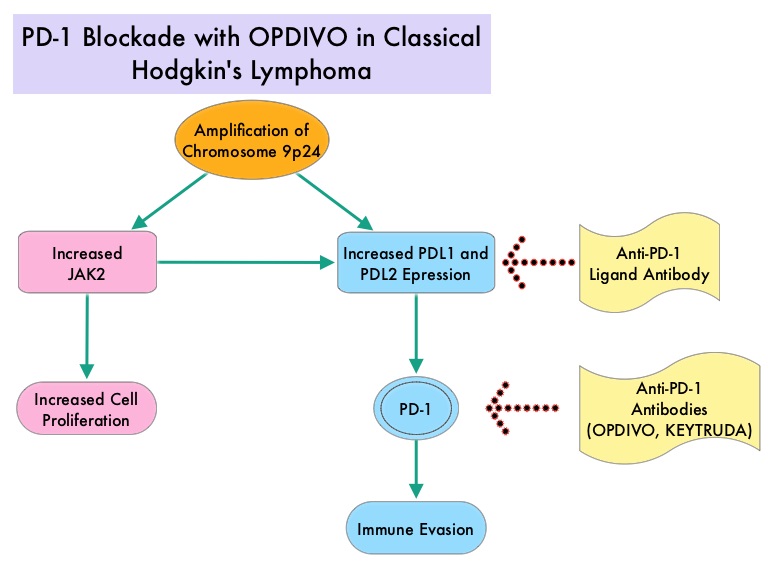 Classical Hodgkin Lymphoma is a malignancy of primarily B lymphocytes and is characterized by the presence of large mononucleated Hodgkin (H) and giant multinucleated Reed-Sternberg (RS) cells collectively known as Hodgkin and Reed-Sternberg cells (HRS). The HRS cells in turn recruit an abundance of ineffective inflammatory cells and infiltrates of immune cells. Preclinical studies suggest that HRS cells evade immune detection by exploiting the pathways associated with immune checkpoint, Programmed Death-1 (PD-1) and its ligands PD-Ls. The most common genetic abnormality in Nodular sclerosis subtype of Hodgkin lymphoma is the selective amplification of genes on the short arm of chromosome 9 (9p24.1) which includes JAK-2 with resulting increased expression of PD-1 ligands such as PDL1 and PDL2 on HRS cells as well as increased JAK-STAT activity, essential for the proliferation and survival of Hodgkin Reed-Sternberg (HRS) cells. Infection with Epstein–Barr virus (EBV) similarly can increase the expression of PDL1 and PDL2 in EBV-positive Hodgkin's lymphomas. It would therefore seem logical to block/inhibit immune check point PD-1 rather than both its ligands, PDL1 and PDL2. Immune checkpoints are cell surface inhibitory proteins/receptors that are expressed on activated T cells. They harness the immune system and prevent uncontrolled immune reactions. Survival of cancer cells in the human body may be to a significant extent related to their ability to escape immune surveillance by inhibiting T lymphocyte activation. The T cells of the immune system therefore play a very important role in modulating the immune system. Under normal circumstances, inhibition of an intense immune response and switching off the T cells of the immune system, is an evolutionary mechanism and is accomplished by Immune checkpoints or gate keepers. With the recognition of Immune checkpoint proteins and their role in suppressing antitumor immunity, antibodies are being developed that target the membrane bound inhibitory Immune checkpoint proteins/receptors such as CTLA-4 (Cytotoxic T-Lymphocyte Antigen 4, also known as CD152), PD-1(Programmed cell Death 1), etc. By doing so, one would expect to unleash the T cells, resulting in T cell proliferation, activation and a therapeutic response.
Classical Hodgkin Lymphoma is a malignancy of primarily B lymphocytes and is characterized by the presence of large mononucleated Hodgkin (H) and giant multinucleated Reed-Sternberg (RS) cells collectively known as Hodgkin and Reed-Sternberg cells (HRS). The HRS cells in turn recruit an abundance of ineffective inflammatory cells and infiltrates of immune cells. Preclinical studies suggest that HRS cells evade immune detection by exploiting the pathways associated with immune checkpoint, Programmed Death-1 (PD-1) and its ligands PD-Ls. The most common genetic abnormality in Nodular sclerosis subtype of Hodgkin lymphoma is the selective amplification of genes on the short arm of chromosome 9 (9p24.1) which includes JAK-2 with resulting increased expression of PD-1 ligands such as PDL1 and PDL2 on HRS cells as well as increased JAK-STAT activity, essential for the proliferation and survival of Hodgkin Reed-Sternberg (HRS) cells. Infection with Epstein–Barr virus (EBV) similarly can increase the expression of PDL1 and PDL2 in EBV-positive Hodgkin's lymphomas. It would therefore seem logical to block/inhibit immune check point PD-1 rather than both its ligands, PDL1 and PDL2. Immune checkpoints are cell surface inhibitory proteins/receptors that are expressed on activated T cells. They harness the immune system and prevent uncontrolled immune reactions. Survival of cancer cells in the human body may be to a significant extent related to their ability to escape immune surveillance by inhibiting T lymphocyte activation. The T cells of the immune system therefore play a very important role in modulating the immune system. Under normal circumstances, inhibition of an intense immune response and switching off the T cells of the immune system, is an evolutionary mechanism and is accomplished by Immune checkpoints or gate keepers. With the recognition of Immune checkpoint proteins and their role in suppressing antitumor immunity, antibodies are being developed that target the membrane bound inhibitory Immune checkpoint proteins/receptors such as CTLA-4 (Cytotoxic T-Lymphocyte Antigen 4, also known as CD152), PD-1(Programmed cell Death 1), etc. By doing so, one would expect to unleash the T cells, resulting in T cell proliferation, activation and a therapeutic response.  The authors in this study enrolled 23 patients with relapsed or refractory Hodgkin's lymphoma of whom 87% had received three or more prior therapies. Seventy eight percent (78%) of the patients had previously received ADCETRIS® (Brentuximab) and 78% had undergone autologous stem-cell transplantation. All patients with the exception of one patient had the nodular-sclerosis subtype of Hodgkin's lymphoma. The median age was 35 years. Patients received OPDIVO® (Nivolumab) at a dose of 3 mg/kg every 2 weeks and treatment was continued until patients had a complete response, tumor progression, or severe toxicities. OPDIVO® is an immune checkpoint PD-1 (Programmed cell Death 1) targeted, fully human, immunoglobulin G4 monoclonal antibody. The median number of OPDIVO® doses that patients received was 16 and the median duration of treatment was 36 weeks. The authors noted an Objective Response Rate of 87% with a complete response of 17%, and a partial response of 70%, in this heavily pretreated group of patients. Stable disease was noted in 13% of the patients. The Progression Free Survival at 24 weeks was 86%.Most of the adverse events were grade 1 and grade 2. Analyses of pretreatment tumor specimens revealed increased expression of PDL1 and PDL2. Reed–Sternberg cells showed nuclear positivity of phosphorylated STAT3, suggestive of active JAK-STAT signaling. The authors concluded that OPDIVO® is highly active with favorable toxicities in heavily pretreated relapsed or refractory Hodgkin's lymphoma “highlighting the genetically defined sensitivity to PD-1 blockade”. PD-1 Blockade with Nivolumab in Relapsed or Refractory Hodgkin's Lymphoma. Ansell SM, Lesokhin AM, Borrello I, et al. N Engl J Med 2015; 372:311-319
The authors in this study enrolled 23 patients with relapsed or refractory Hodgkin's lymphoma of whom 87% had received three or more prior therapies. Seventy eight percent (78%) of the patients had previously received ADCETRIS® (Brentuximab) and 78% had undergone autologous stem-cell transplantation. All patients with the exception of one patient had the nodular-sclerosis subtype of Hodgkin's lymphoma. The median age was 35 years. Patients received OPDIVO® (Nivolumab) at a dose of 3 mg/kg every 2 weeks and treatment was continued until patients had a complete response, tumor progression, or severe toxicities. OPDIVO® is an immune checkpoint PD-1 (Programmed cell Death 1) targeted, fully human, immunoglobulin G4 monoclonal antibody. The median number of OPDIVO® doses that patients received was 16 and the median duration of treatment was 36 weeks. The authors noted an Objective Response Rate of 87% with a complete response of 17%, and a partial response of 70%, in this heavily pretreated group of patients. Stable disease was noted in 13% of the patients. The Progression Free Survival at 24 weeks was 86%.Most of the adverse events were grade 1 and grade 2. Analyses of pretreatment tumor specimens revealed increased expression of PDL1 and PDL2. Reed–Sternberg cells showed nuclear positivity of phosphorylated STAT3, suggestive of active JAK-STAT signaling. The authors concluded that OPDIVO® is highly active with favorable toxicities in heavily pretreated relapsed or refractory Hodgkin's lymphoma “highlighting the genetically defined sensitivity to PD-1 blockade”. PD-1 Blockade with Nivolumab in Relapsed or Refractory Hodgkin's Lymphoma. Ansell SM, Lesokhin AM, Borrello I, et al. N Engl J Med 2015; 372:311-319