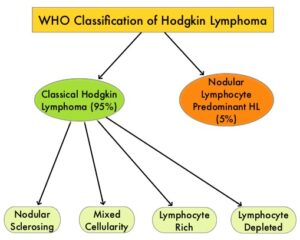
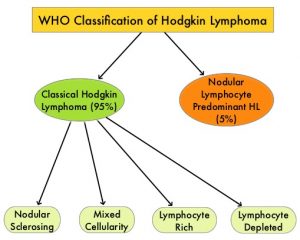
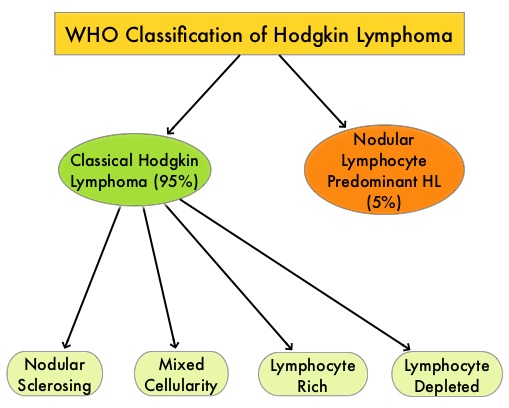
SUMMARY: The American Cancer Society estimates that in the United States for 2024, about 8570 new cases of Hodgkin Lymphoma will be diagnosed and about 910 patients will die of the disease. Hodgkin Lymphoma is classified into two main groups – Classical Hodgkin Lymphomas and Nodular Lymphocyte Predominant type, by the World Health Organization. The Classical Hodgkin Lymphomas include Nodular sclerosing, Mixed cellularity, Lymphocyte rich, Lymphocyte depleted, subtypes and accounts for approximately 10% of all malignant lymphomas. Nodular sclerosis Hodgkin lymphoma histology, accounts for approximately 80% of Hodgkin Lymphoma cases in older children and adolescents in the United States. Classical Hodgkin Lymphoma is a malignancy of primarily B lymphocytes and is characterized by the presence of large mononucleated Hodgkin and giant multinucleated Reed-Sternberg (RS) cells collectively known as Hodgkin and Reed-Sternberg cells (HRS).
For patients with Hodgkin Lymphoma, the goal of first-line chemotherapy is cure. A positive PET scan following first-line chemotherapy is indicative of incomplete response with residual disease and warrants subsequent chemotherapy or radiation. Advanced stage (Stage III-IV) Classical Hodgkin lymphoma has a cure rate of approximately 70-80% when treated in the first-line setting with a combination of Doxorubicin, Bleomycin, Vinblastine, and Dacarbazine (ABVD). This regimen which was developed more than 40 years ago is less expensive, easy to administer, is generally well tolerated and is often used in first line setting. Nonetheless, this regimen which contains Bleomycin can cause pulmonary toxicity, the incidence of which is higher in older patients and in those who receive consolidation radiotherapy to the thorax.
Brentuximab Vedotin (ADCETRIS®) is an Antibody-Drug Conjugate (ADC) that targets CD30, which is a surface antigen, expressed on Reed-Sternberg cells, in patients with Classical Hodgkin lymphoma. This ADC consists of an anti-CD30 monoclonal antibody linked to MonoMethyl Auristatin E (MMAE), an antimicrotubule agent. Upon binding to the CD30 molecule on the cancer cells, MMAE is released into the cancer cell, resulting in cell death. In the ECHELON-1 study, frontline treatment with Brentuximab Vedotin (BV) in combination with Doxorubicin, Vinblastine and Dacarbazine (AVD) resulted in a significant improvement both in Progression Free Survival as well as Overall Survival, after a median follow up of 6 years. However, frontline BV adds toxicity, and 7-20% of patients still develop Relapsed/Refractory Hodgkin Lymphoma.
Preclinical studies suggest that HRS cells evade immune detection by exploiting the pathways associated with immune checkpoint, Programmed Death-1 (PD-1) and its ligands PD-L. Classical Hodgkin Lymphoma is an excellent example of how the tumor microenvironment influences cancer cells to proliferate and survive. The most common genetic abnormality in Nodular sclerosis subtype of Hodgkin lymphoma is the selective amplification of genes on the short arm of chromosome 9 (9p24.1) which includes JAK-2, with resulting increased expression of PD-1 ligands such as PDL1 and PDL2 on HRS cells, as well as increased JAK-STAT activity, essential for the proliferation and survival of Hodgkin Reed-Sternberg (HRS) cells. Infection with Epstein–Barr virus (EBV) similarly can increase the expression of PDL1 and PDL2 in EBV-positive Hodgkin lymphomas. It would therefore seem logical to block or inhibit immune check point PD-1 rather than both its ligands, PDL1 and PDL2.
Nivolumab (OPDIVO®) is a fully human, immunoglobulin G4 monoclonal antibody that binds to the PD-1 receptor and blocks its interaction with PD-L1 and PD-L2, thereby undoing PD-1 pathway-mediated inhibition of the immune response and unleashing the T cells.
SWOG S1826 was an open-label, randomized Phase III trial conducted to compare the combination of Nivolumab plus AVD to Brentuximab Vedotin plus AVD, in patients with advanced-stage classical Hodgkin Lymphoma (cHL). In this study, 976 newly diagnosed Stage III or IV cHL patients (N=976) were randomly assigned 1:1 to receive either 6 cycles of Nivolumab at 240 mg IV on days 1 and 15 (N=489) or Brentuximab Vedotin 1.2 mg/kg IV on days 1 and 15 (N=487). Both treatment groups also received AVD IV (Doxorubicin, Vinblastine, Dacarbazine ) on days 1 and 15, and treatment was repeated every 28 days for 6 cycles in the absence of disease progression or unacceptable toxicity. Granulocyte-Colony Stimulating Factor (G-CSF) Pegfilgrastim SC on days 2 and 16, or Filgrastim SC on days 6-10 and 21-25 was optional in the Nivolumab group (N-AVD) but was required in the Brentuximab Vedotin group (BV-AVD). Approximately 54% in the N-AVD group received G-CSF compared to 95% in the BV-AVD group. After completion of cycle 6, patients could receive radiation therapy at the discretion of the treating physician, to metabolically active residual lesions noted on the end of treatment PET. Less than 1% of patients had received radiotherapy. Patients were stratified by age, International Prognostic Score (IPS) and intent to use radiation therapy. The median age was 27 years, 76% were Caucasian, 55% were men, 64% had Stage IV disease and 32% had IPS of 4-7. The Primary endpoint was Progression Free Survival (PFS). Secondary endpoints included Overall Survival (OS), Event-Free Survival (EFS), Patient-Reported Outcomes (PROs), and Safety.
At the planned 2nd interim analysis, upon recommendation from the SWOG Data and Safety Monitoring Committee, the primary results were reported. With a median follow up of 12.1 months, PFS was superior in the N-AVD group compared to the BV-AVD group (HR=0.48; P=0.001). The 1 year PFS was 94% in the N-AVD group compared with 86% among patients treated with BV-AVD.
The researchers repeated the analysis after an additional one year of follow up, to assess the durability of PFS benefit. At a median follow-up of 2.1 years, the 2-year PFS was 92% with N-AVD compared to 83% with BV-AVD (HR=0.45). The PFS benefit was consistent across prespecified treatment subgroups, including subgroups according to age, disease stage and IPS score. The 2-year EFS was 90% with N-AVD versus 81% with BV-AVD (HR for death=0.50). More importantly, N-AVD was associated with favorable side-effect profile with a lower frequency of neurotoxicities. Further, there was a dramatic reduction in the use of radiation in adolescent patients.
The rate of Grade 3 or more hematologic AEs were 48.4% after N-AVD, compared to 30.5% after BV-AVD. There was however no increase in infectious complications even though there was a higher rate of neutropenia in the N-AVD group. It should be noted that the frequency of neutropenia with BV-AVD was ameliorated by the required use of G-CSF, as compared with the optional use of G-CSF with N-AVD. Hypo/Hyperthyroidism was more frequent after N-AVD whereas peripheral neuropathy was more common after BV-AVD.
The researchers concluded that in this largest Hodgkin Lymphoma study in National Clinical Trials Network (NCTN) history, Nivolumab in combination with AVD significantly improved Progression Free Survival with fewer toxicities, compared to Brentuximab Vedotin in combination with AVD, in patients with advanced stage Hodgkin Lymphoma. Nivolumab in combination with AVD may be the new standard therapy for this group of patients. Follow-up is ongoing to confirm the durability of PFS benefit, and to assess Overall Survival and Patient Reported Outcomes.
Nivolumab+AVD in Advanced-Stage Classic Hodgkin’s Lymphoma. Herrera AF, LeBlanc M, Castellino SM, et al. N Engl J Med 2024;391:1379-1389.