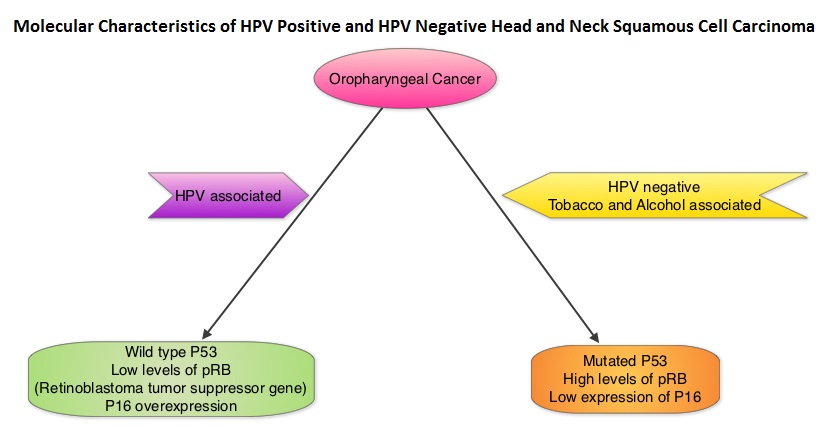
SUMMARY: The American Cancer Society estimates that in the US for 2022, about 54,000 new cases of oral cavity or oropharyngeal cancer will be diagnosed and about 11,230 patients will die of the disease. Patients with Squamous Cell Carcinoma of the head and neck, frequently present with locoregionally advanced disease.
The treatment paradigm for Head and Neck cancer has been rapidly evolving with the recognition and better understanding of immune evasion and the role of immune checkpoints or gate keepers in suppressing antitumor immunity. Blocking the immune checkpoints unleashes the T cells, resulting in T cell proliferation, activation, and a therapeutic response. Checkpoint inhibitors administered in a neoadjuvant setting activates both the priming phase of immunity within tumor tissue, and the effector phase within the tumor microenvironment. It has been shown that neoadjuvant immunotherapy expands more T-cell clones than adjuvant treatment. Preclinical models have also demonstrated that both radiation therapy and Cisplatin chemotherapy increase the PD-L1 expression on the tumor, suggesting that combining radiotherapy with anti-PD-1 therapy could improve the outcomes.
Pembrolizumab (KEYTRUDA®) is a fully humanized, Immunoglobulin G4, monoclonal antibody and checkpoint inhibitor, that binds to the PD-1 receptor and blocks its interaction with ligands PD-L1 and PD-L2, thereby undoing PD-1 pathway-mediated inhibition of the immune response and unleashing the tumor-specific effector T cells. Pembrolizumab has been shown to improve Overall Survival in patients with Recurrent/Metastatic Head and Neck Squamous Cell Carcinoma
KEYNOTE-412 is a randomized, double-blind, Phase III trial, conducted to evaluate the efficacy and safety of Pembrolizumab in combination with chemoradiation versus placebo in combination with chemoradiation, in treatment naïve patients with locally advanced Head and Neck Squamous Cell carcinoma. In this study, 804 patients were randomly assigned 1:1 to receive Pembrolizumab 200 mg IV every 3 weeks plus chemoradiation (70Gy in 35 fractions along with Cisplatin 100 mg/m2 IV every 3 weeks) followed by Pembrolizumab (N=402), or placebo every 3 weeks plus chemoradiation, followed by placebo (N=402). Patients received Pembrolizumab /placebo priming dose 1 week before chemoradiation, followed by 2 doses during chemoradiation and 14 doses of maintenance therapy after chemoradiation, for a total of 17 doses. Enrolled patients had newly diagnosed, pathologically proven, treatment naive locally advanced Head and Neck Squamous Cell carcinoma (T3-T4, N0-N3 or any N2a-3, T1-T4 larynx/hypopharynx/oral cavity/p16-negative oropharynx cancers, or T4 or N3 p16-positive oropharynx cancer). Both treatment groups were well balanced. The Primary endpoint was Event Free Survival (EFS). Secondary endpoints included Overall Survival (OS), and Safety.
At the time of data cutoff, with a median follow up of 47.7 months, there was a favorable trend toward improved Event Free Survival (EFS) with the addition of Pembrolizumab vs placebo to chemoradiation (HR 0.83, P=0.04), but the difference did not achieve statistical significance. The 2-year EFS was 63.2% in the Pembrolizumab group and 56.2% in the placebo group. In an exploratory analysis however, the 2-year EFS among patients with high expression of PD-L1 (CPS 20 or higher) was 71% in the Pembrolizumab group and 62% in the placebo group. A favorable of Overall Survival benefit was also observed among these patients, with a 3-year OS of 79% in Pembrolizumab group and 73% in the placebo group.
It was concluded that Pembrolizumab in combination with chemoradiation was associated with a favorable trend toward improved Event Free Survival, compared with placebo plus chemoradiation, in patients with locally advanced Head and Neck Squamous Cell carcinoma, but the difference did not reach statistical significance. The researchers added that perhaps patients with high CPS score on the tumor could benefit with this treatment approach.
Primary results of the phase III KEYNOTE-412 study: Pembrolizumab (pembro) with chemoradiation therapy (CRT) vs placebo plus CRT for locally advanced (LA) head and neck squamous cell carcinoma (HNSCC). Machiels J, Tao Y, Burtness B, et al. Annals of Oncology (2022) 33 (suppl_7): S808-S869. 10.1016/annonc/annonc1089. LBA5

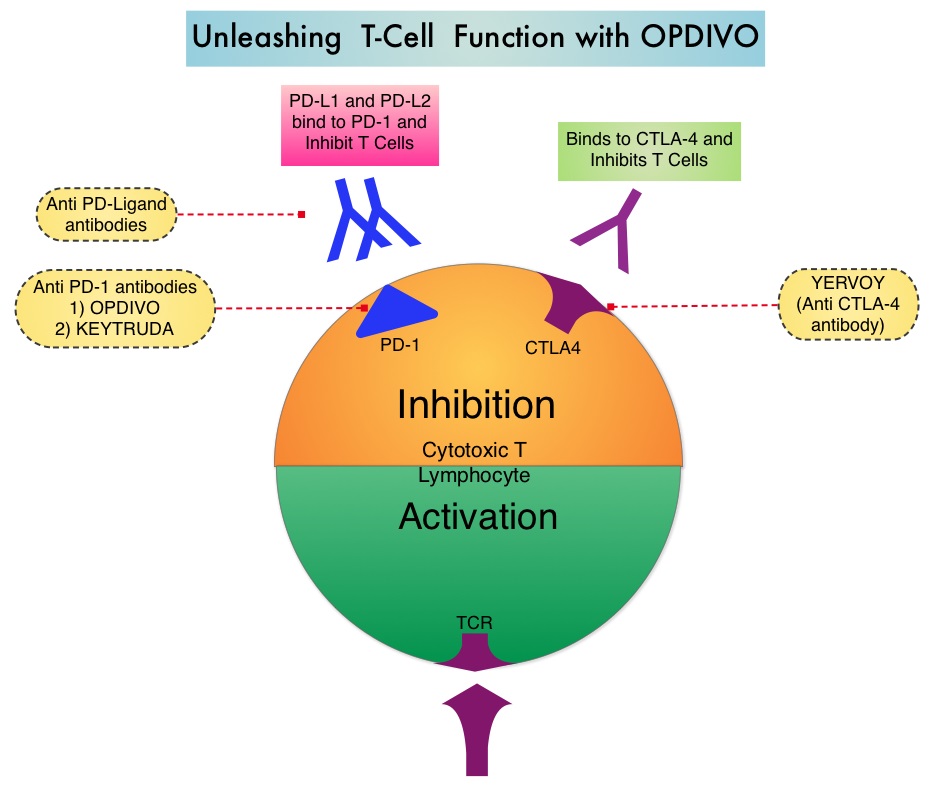
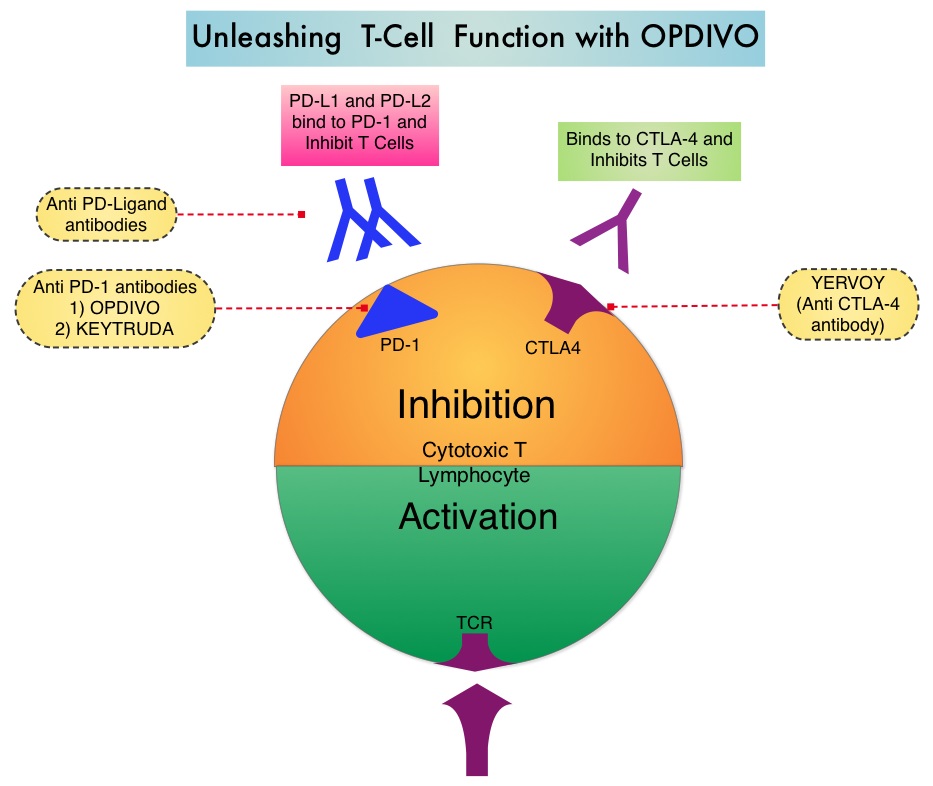
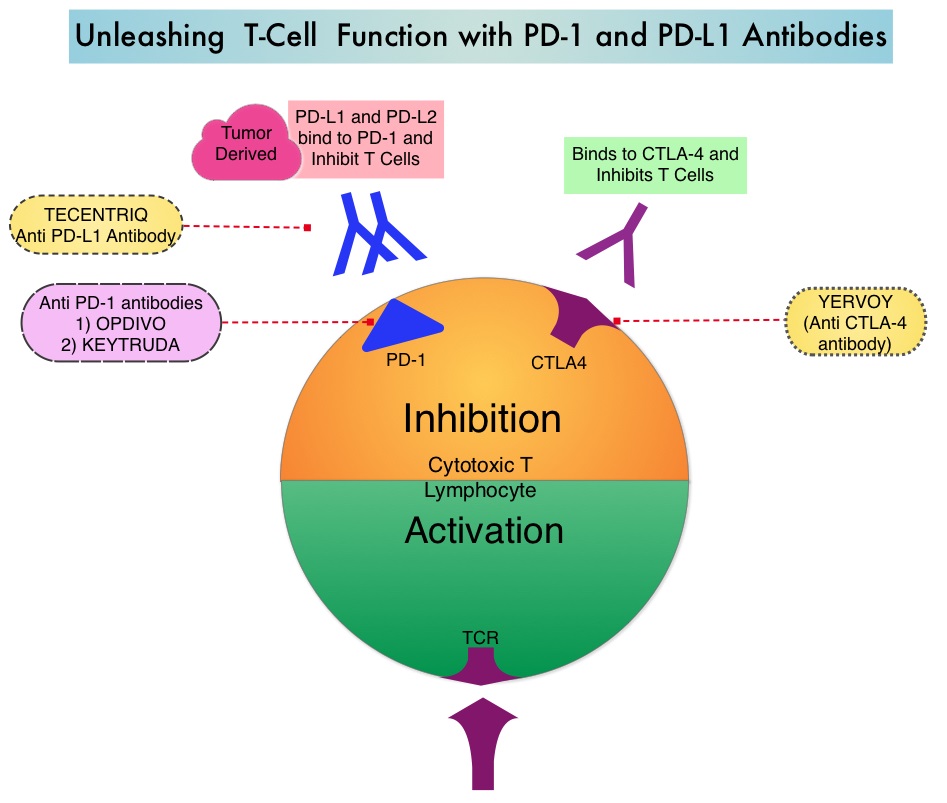 The treatment paradigm for solid tumors has been rapidly evolving with a better understanding of the Immune checkpoints or gate keepers. Immune checkpoints are cell surface inhibitory proteins/receptors that are expressed on activated T cells. They harness the immune system and prevent uncontrolled immune reactions. Survival of cancer cells in the human body may be to a significant extent related to their ability to escape immune surveillance by inhibiting T lymphocyte activation. The T cells of the immune system therefore play a very important role in modulating the immune system. Under normal circumstances, inhibition of an intense immune response and switching off the T cells of the immune system, is an evolutionary mechanism and is accomplished by Immune checkpoints or gate keepers. With the recognition of Immune checkpoint proteins and their role in suppressing antitumor immunity, antibodies are being developed that target the membrane bound inhibitory Immune checkpoint proteins/receptors such as CTLA-4 (Cytotoxic T-Lymphocyte Antigen 4, also known as CD152), PD-1(Programmed cell Death 1), etc. By doing so, one would expect to unleash the T cells, resulting in T cell proliferation, activation and a therapeutic response.
The treatment paradigm for solid tumors has been rapidly evolving with a better understanding of the Immune checkpoints or gate keepers. Immune checkpoints are cell surface inhibitory proteins/receptors that are expressed on activated T cells. They harness the immune system and prevent uncontrolled immune reactions. Survival of cancer cells in the human body may be to a significant extent related to their ability to escape immune surveillance by inhibiting T lymphocyte activation. The T cells of the immune system therefore play a very important role in modulating the immune system. Under normal circumstances, inhibition of an intense immune response and switching off the T cells of the immune system, is an evolutionary mechanism and is accomplished by Immune checkpoints or gate keepers. With the recognition of Immune checkpoint proteins and their role in suppressing antitumor immunity, antibodies are being developed that target the membrane bound inhibitory Immune checkpoint proteins/receptors such as CTLA-4 (Cytotoxic T-Lymphocyte Antigen 4, also known as CD152), PD-1(Programmed cell Death 1), etc. By doing so, one would expect to unleash the T cells, resulting in T cell proliferation, activation and a therapeutic response.