SUMMARY: The American Cancer Society estimates that about 9,280 new CML cases will be diagnosed in the United States in 2024 and about 1,280 patients will die of the disease. Chronic Myeloid Leukemia (CML) constitutes about 15% of all new cases of leukemia and the average age at diagnosis of CML is around 64 years. The hallmark of CML, the Philadelphia Chromosome (Chromosome 22), is a result of a reciprocal translocation between chromosomes 9 and 22, wherein the ABL gene from chromosome 9 fuses with the BCR gene on chromosome 22. As a result, the auto inhibitory function of the ABL gene is lost and the BCR-ABL fusion gene is activated resulting in cell proliferation and leukemic transformation of hematopoietic stem cells.
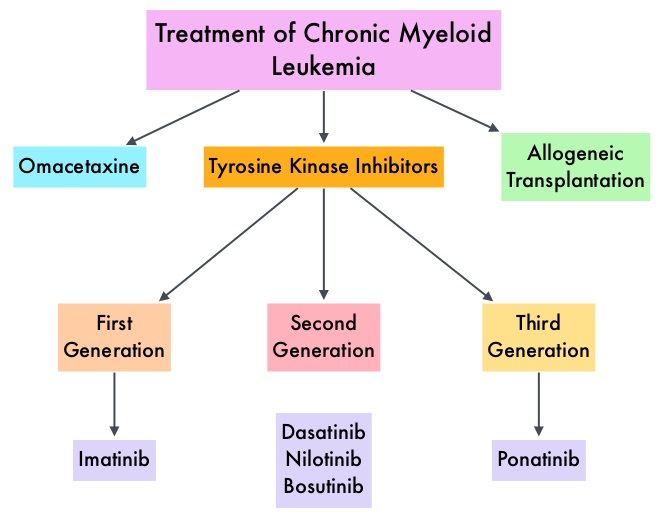
The Tyrosine Kinase Inhibitors (TKIs) approved for newly diagnosed chronic phase CML in the United States share the same therapeutic target, which is the ATP-binding site of BCR-ABL1 kinase. They include first-generation TKI Imatinib (GLEEVEC®) or second-generation TKIs Nilotinib (TASIGNA®), Dasatinib (SPRYCEL®), or Bosutinib (BOSULIF®). Imatinib is associated with lower patient response and a higher incidence of disease progression than those with second-generation TKIs, whereas treatment with second-generation TKIs can result in faster, deeper molecular responses than Imatinib in frontline therapy, but are associated with more adverse events, necessitating dose modifications and switching treatments. Further, close to 50% of clinical resistance is associated with the acquisition of mutations in this region of the kinase, resulting in conformational changes that render TKIs inactive. Therefore resistance to one of the TKIs, will likely result in resistance to the others as well. Further, the “gatekeeper” T315I mutation, which has been reported in 20% of patients with mutations, confers resistance to all clinically available TKIs except Ponatinib (ICLUSIG®). There is therefore an unmet need for a safe and effective frontline therapy for patients with newly diagnosed chronic phase CML
Asciminib (SCEMBLIX®) is a novel, first-in-class, potent and specific, oral BCR-ABL1 inhibitor that does not bind to the ATP-binding site of the kinase. Instead, it specifically targets the ABL1 myristoyl pocket, also known as a STAMP (Specifically Targeting the ABL Myristoyl Pocket) inhibitor, with activity against native unmutated BCR-ABL1, and all clinically observed ATP-site mutants, including T315I. In a Phase I study, Asciminib was active in heavily pretreated patients with CML who had resistance to or unacceptable side effects from TKIs, including patients in whom Ponatinib had failed, and those with a T315I mutation.
Asciminib is approved in the US for the treatment of adults with Philadelphia Chromosome positive chronic phase CML who have previously been treated with two or more TKIs. It is also approved in patients with Philadelphia Chromosome positive chronic phase CML with the T315I mutation.
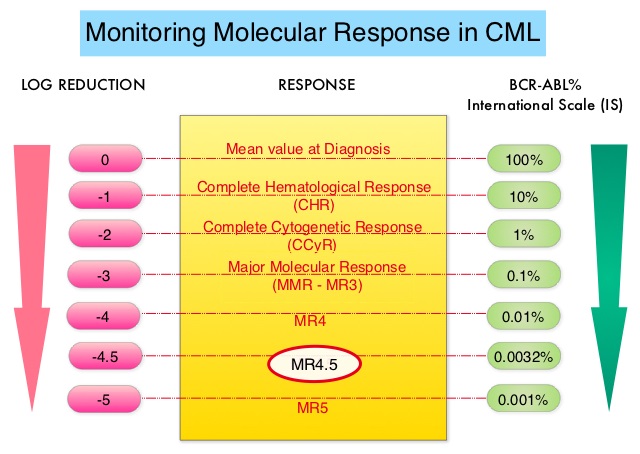
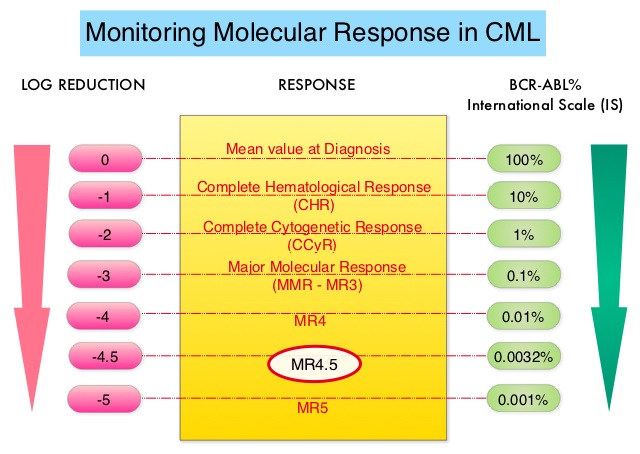
The ASC4FIRST study is a pivotal Phase III, multi-center, open-label, randomized trial aimed at evaluating the efficacy and safety of Asciminib compared to investigator-selected Tyrosine Kinase Inhibitors (TKIs) in adult patients with newly diagnosed Philadelphia chromosome positive Chronic Myeloid Leukemia in chronic phase (CML-CP). A total of 405 patients were enrolled and were randomly assigned in a 1:1 ratio to receive either Asciminib 80 mg orally once daily (N=201) or investigator-selected TKIs which included Imatinib and second generation TKIs such as Bosutinib, Dasatinib or Nilotinib given at approved doses (N=204). Before randomization, investigators after discussing with patients selected a TKI (either Imatinib or one of the second-generation TKIs a patient would take, if randomly assigned to the comparator group-prerandomization selected TKI), considering treatment goals, disease and patient characteristics, and coexisting conditions. Randomization was stratified by European Treatment and Outcome Study long-term survival score category (low, intermediate, or high risk), and by TKI selected by investigators before randomization. The two Primary objectives of this study were to compare the efficacy of Asciminib with that of investigator-selected TKIs (all members of this class considered together as a group), and to compare the efficacy of Asciminib with that of Imatinib. Asciminib was not compared with second-generation TKIs as a primary objective. The Primary end point for both objectives was Major Molecular Response (defined as BCR/ABL1 transcript levels 0.1% or less on the International Scale at week 48 that did not meet any treatment failure criteria. The Secondary objective of this study was assessment of Major Molecular Response (MMR) at week 48 with Asciminib, as compared with investigator-selected TKIs among patients with second-generation TKIs as their prerandomization-selected TKI. The median follow-up was 16.3 months in the Asciminib group and 15.7 months in the investigator-selected TKI group.
At the 48-week mark, Asciminib demonstrated a significantly higher MMR rate compared to investigator-selected TKIs (67.7% versus 49.0%; P<0.001). Deep molecular response rates (BCR/ABL1 transcript levels 0.01% or less, were also superior in the Asciminib group compared to investigator-selected TKIs (38.8% versus 20.6%). Patients preselected for Imatinib who were randomized to Asciminib achieved an MMR rate of 69.3% compared to 40.2% in the Imatinib group. Among those preselected for second-generation TKIs, the MMR rate was 66.0% for Asciminib versus 57.8% for the second-generation TKI group.
Asciminib exhibited a favorable safety profile with fewer Grade 3 or higher Adverse Events and lower rates of treatment discontinuation due to Adverse Events. Grade 3 or higher Adverse Events for Asciminib was 38%, for Imatinib was 44.4% and for second-generation TKIs was 54.9%. Discontinuation due to Adverse Events for Asciminib was 4.5%, for Imatinib was 11.1% and for Second-generation TKIs was 9.8%.
It was concluded that Asciminib is the only agent to demonstrate superiority over investigator selected standard-of-care TKIs in achieving higher MMR rates at 48 weeks in newly diagnosed chronic phase CML patients, alongside a better safety and tolerability profile. These findings indicate that Asciminib could significantly improve the treatment landscape for this group of patients, offering hope for better disease control and quality of life, thereby addressing key unmet needs in CML management.
ASC4FIRST, a pivotal phase 3 study of asciminib (ASC) vs investigator-selected tyrosine kinase inhibitors (IS TKIs) in newly diagnosed patients (pts) with chronic myeloid leukemia (CML): Primary results. Hughes TP, Hochhaus A, Takahashi N, et al. J Clin Oncol 42, 2024 (suppl 17; abstr LBA6500)

 This new approval for BOSULIF® was based on the efficacy and safety data from BFORE trial, which is an ongoing randomized, open-label, multicenter, phase III study in which in 487 patients with Ph+ newly diagnosed Chronic Phase CML were randomized to receive either BOSULIF® 400 mg once daily (N=246) or GLEEVEC® (Imatinib) 400 mg once daily (N=241).
This new approval for BOSULIF® was based on the efficacy and safety data from BFORE trial, which is an ongoing randomized, open-label, multicenter, phase III study in which in 487 patients with Ph+ newly diagnosed Chronic Phase CML were randomized to receive either BOSULIF® 400 mg once daily (N=246) or GLEEVEC® (Imatinib) 400 mg once daily (N=241).