SUMMARY: Multiple Myeloma is a clonal disorder of plasma cells in the bone marrow and the American Cancer Society estimates that in the United States, 34,920 new cases will be diagnosed in 2021 and 12,410 patients are expected to die of the disease. Multiple Myeloma (MM) in 2021 remains an incurable disease. The therapeutic goal therefore is to improve Progression Free Survival (PFS) and Overall Survival (OS). Multiple Myeloma is a disease of the elderly, with a median age at diagnosis of 69 years and characterized by intrinsic clonal heterogeneity. Almost all patients eventually will relapse, and patients with a high-risk cytogenetic profile, extramedullary disease or refractory disease have the worst outcomes. The median survival for patients with myeloma is over 10 years.
DARZALEX® is a human IgG1 antibody that targets CD38, a transmembrane glycoprotein abundantly expressed on malignant plasma cells and with low levels of expression on normal lymphoid and myeloid cells. DARZALEX® exerts its cytotoxic effect on myeloma cells by multiple mechanisms, including Antibody Dependent Cellular Cytotoxicity (ADCC), Complement Mediated Cytotoxicity and direct apoptosis. Additionally, DARZALEX® may have a role in immunomodulation, by depleting CD38-positive regulator Immune suppressor cells, and thereby expanding T cells, in patients responding to therapy. DARZALEX® has activity as both a single agent and when combined with other standard regimens. POMALYST® (Pomalidomide) is a novel, oral, immunomodulatory drug which is far more potent than THALOMID® (Thalidomide) and REVLIMID® (Lenalidomide), and has been shown to be active in REVLIMID® and VELCADE® refractory patients. In the EQUULEUS Phase Ib study, intravenous DARZALEX® in combination with POMALYST® and Dexamethasone in heavily pretreated relapsed or refractory Multiple Myeloma, resulted in a Very Good Partial Response (VGPR) or better in 42% of patients.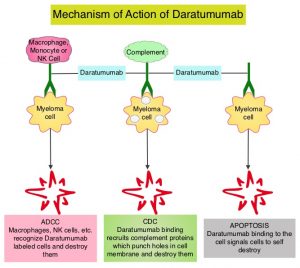
Recently published studies have concluded that the subcutaneous formulation of DARZALEX® resulted in non-inferior pharmacokinetics and efficacy compared to the current IV formulation, and also importantly offers the potential for a fixed-dose administration, shorter administration times and a lower rate of infusion-related reactions with improved safety profile.
APOLLO study is an open-label, randomized, multicenter, Phase III trial, conducted by the European Myeloma Network investigators, to evaluate SubCutaneous (SC) formulation of DARZALEX® in combination with POMALYST® and Dexamethasone (D-Pd; N=151) versus POMALYST® and Dexamethasone (Pd; N=153) alone, in relapsed/refractory Multiple Myeloma patients who have received one or more prior lines of therapy including REVLIMID® and a Proteasome Inhibitor. This study enrolled 304 patients with relapsed or refractory Multiple Myeloma, and prior treatment with anti-CD38 antibody or POMALYST® was not permitted. Treatment for all patients consisted of POMALYST® 4 mg orally daily plus Dexamethasone 40 mg orally on days 1, 8, 15, and 22 (20 mg for patients aged 75 years or older), given every 28 days. Patients in the D-Pd group additionally received DARZALEX® 1800 mg SC co-formulated with recombinant human hyaluronidase PH20 (rHuPH20; ENHANZE® drug delivery technology, Halozyme, Inc.), given weekly for cycles 1 to 2, every 2 weeks for cycles 3 to 6, and every 4 weeks thereafter. The median age was 67 years, and 35% had high cytogenetic risk (presence of del17p, t[14;16], or t[4;14]). The median prior lines of therapy were 2, approximately 80% of patients were refractory to REVLIMID®, 48% of patients were refractory to a Proteosome Inhibitor, and 42% of patients were refractory to both agents. Treatment was continued until disease progression or unacceptable toxicity. The median duration of treatment was 11.5 months with D-Pd, compared with 6.6 months with Pd. The Primary endpoint was Progression Free Survival (PFS). Secondary endpoints included Overall Response Rate (ORR), Very Good Partial Response (VGPR), Complete Response (CR), MRD negativity rate, Overall Survival (OS), and Safety.
The study met its Primary endpoint of improved PFS in the primary analysis at a median follow up of 16.9 months. The median PFS for the D-Pd group was 12.4 months versus 6.9 months for Pd group (HR=0.63; P=0.0018). This represented a 37% reduction in the risk of progression or death in patients treated with D-Pd. Among patients who were refractory to REVLIMID®, median PFS was 9.9 months in the D-Pd group versus 6.5 months in the Pd group. This benefit was seen across all subgroups of patients, regardless of age, stage, prior line of therapy, REVLIMID® refractoriness and cytogenetic risk. D-Pd regimen was also superior to Pd regimen in terms of other endpoints, including ORR (69% versus 46%), VGPR or better (51% versus 20%), CR (25% versus 4%), and MRD negativity (9% versus 2%). Survival data are immature and follow up is ongoing. Infusion-related events were rare, and seen in 6% of patients treated with D-Pd, and local injection-site reactions which were all Grade 1 were seen in 2% of patients in the D-Pd group. Treatment discontinuation due to treatment-related adverse events, were similar for the D-Pd and Pd groups (2% versus 3%).
It was concluded that Subcutaneous DARZALEX® given along with POMALYST® and Dexamethasone significantly reduced the risk of progression or death by 37% in patients with relapsed/refractory Multiple Myeloma, compared to POMALYST® and Dexamethasone alone. The infusion-related reaction rate was very low and median duration of injection administration was short at 5 minutes. Subcutaneous DARZALEX® thus has a high likelihood of changing clinical practice, increasing convenience for patients and decreasing treatment burden.
Daratumumab plus pomalidomide and dexamethasone versus pomalidomide and dexamethasone alone in previously treated multiple myeloma (APOLLO): an open-label, randomised, phase 3 trial. Dimopoulos MA, Terpos E, Boccadoro M, et al. Lancet Oncol. 2021;22:801-812. doi:10.1016/S1470-2045(21)00128-5

