SUMMARY: Prostate cancer is the most common cancer in American men with the exclusion of skin cancer and 1 in 7 men will be diagnosed with prostate cancer during their lifetime. It is estimated that in the United States, about 161,360 new cases of prostate cancer will be diagnosed in 2017 and 26,730 men will die of the disease. The development and progression of prostate cancer is driven by androgens. Androgen Deprivation Therapy (ADT) has therefore been the cornerstone of treatment of advanced prostate cancer and is the first treatment intervention for hormone sensitive prostate cancer. Chemotherapy is usually considered for patients who progress on hormone therapy (Castrate Resistant Prostate Cancer-CRPC) and TAXOTERE® (Docetaxel) has been shown to improve Overall Survival (OS) of metastatic prostate cancer patients, who had progressed on Androgen Deprivation Therapy. Tumors in patients with CRPC are not androgen independent and continue to rely on Androgen Receptor signaling. Two oral Androgen Receptor Targeted Agents (ARTA) are presently available for metastatic CRPC. They include ZYTIGA® (Abiraterone) and XTANDI® (Enzalutamide). ZYTIGA® inhibits CYP 17A1 enzyme thus decreasing androgen biosynthesis and depletes adrenal and intratumoral androgens. XTANDI® competes with Testosterone and Dihydrotestosterone and avidly binds to the Androgen Receptor (AR), thereby inhibiting AR signaling and in addition inhibits translocation of the AR into the nucleus and thus inhibits the transcriptional activities of the AR. There is presently very little guidance with regards to the sequencing of these two oral agents.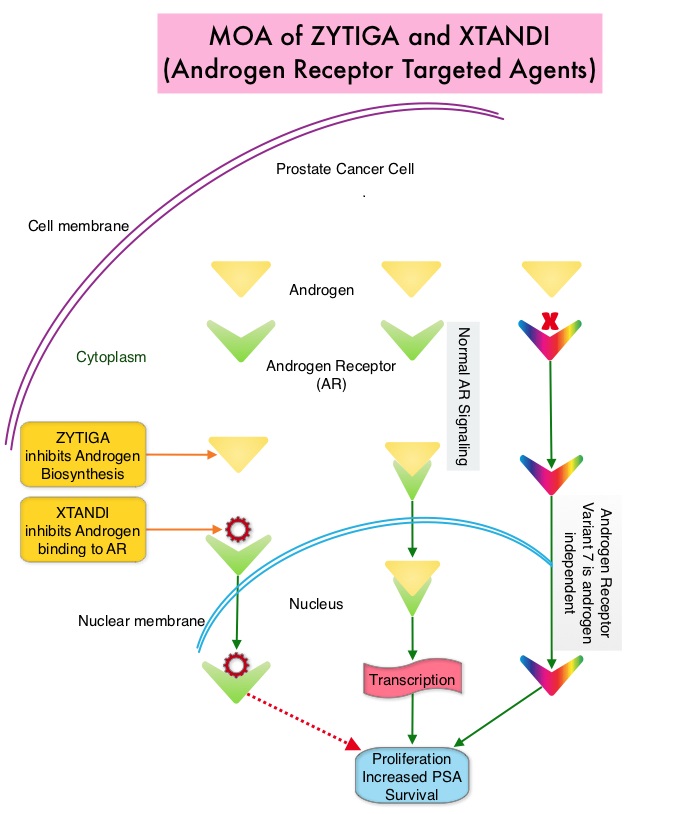
It has remained unclear when a patient should receive chemotherapy following progression on AR-targeted therapies. To determine the ideal second line therapy in this patient population, the authors conducted a retrospective study, to assess if second line chemotherapy was associated with improved outcomes, compared with second line alternative AR Targeted Agents, in patients with a very short duration of response to first line Androgen Receptor Targeted Agents, in the US community oncology setting.
Using Altos electronic medical records, the authors identified 345 patients with metastatic CRPC who did not respond to first-line AR Targeted Agents (ZYTIGA® or XTANDI®) who then went on to receive second-line TAXOTERE® – Docetaxel or JEVTANA® – Cabazitaxel (chemotherapy cohort, N=147), or an alternative AR Targeted Agent (ARTA cohort, N=198), from May 2011 to Oct 2014. Patients receiving chemotherapy as second-line treatment, compared to those receiving second-line ARTA, were younger (median age, 74 vs 79 years) and had several poor prognostic factors including a higher mean PSA, LDH and Alkaline Phosphatase, as well as lower mean hemoglobin and increased opioid use. Treatment outcomes were evaluated from start of second-line treatment and second-line chemotherapy was compared to second-line ARTA. The primary endpoints included Prostate Specific Antigen (PSA) response (50% or more reduction) and Overall Survival (OS).
It was noted that more patients in the chemotherapy group had a PSA response compared to the AR Targeted Agent group (P=0.005), and there was a non-statistically significant trend toward improved Overall Survival for second-line chemotherapy versus AR Targeted Agent (adjusted HR=0.81; P=0.15). Among patients with poor prognostic features, those in the chemotherapy cohort had significantly improved Overall Survival (adjusted HR=0.55; P=0.009) compared with those in the AR Targeted Agent cohort.
The authors concluded that patients who do not respond well to first-line Androgen Receptor Targeted Agent and have poor prognostic features, should not receive a second AR-targeted agent but instead receive second-line chemotherapy, as this may confer a survival benefit. Real-world outcomes in patients with metastatic castration-resistant prostate cancer receiving second-line chemotherapy vs alternative androgen receptor-target agents (ARTA) after lack of response to first-line ARTA in US community oncology practices. Oh WK, Cheng WY, Miao R, et al. J Clin Oncol. 2017;35 (suppl 6S; abstr 214).

