SUMMARY: The American Cancer Society estimates that in 2018, about 74,680 people will be diagnosed with Non Hodgkin Lymphoma (NHL) in the United States and about 19,910 individuals will die of this disease. Indolent Non Hodgkin Lymphomas are mature B cell lymphoproliferative disorders and include Follicular Lymphoma, Nodal Marginal Zone Lymphoma (NMZL), Extranodal Marginal Zone Lymphoma (ENMZL) of Mucosa-Associated Lymphoid Tissue (MALT), Splenic Marginal Zone Lymphoma (SMZL), LymphoPlasmacytic Lymphoma (LPL) and Small Lymphocytic Lymphoma (SLL).
Follicular Lymphoma is the most indolent form and second most common form of all NHLs and they are a heterogeneous group of lymphoproliferative malignancies. Approximately 20% of all NHLs are Follicular Lymphomas. Advanced stage indolent NHL is not curable and as such, prolonging Progression Free Survival (PFS) and Overall Survival (OS), while maintaining Quality of Life, have been the goals of treatment intervention. Asymptomatic patients with indolent NHL are generally considered candidates for “watch and wait” approach. Patients with advanced stage symptomatic Follicular Lymphoma are often treated with induction chemoimmunotherapy followed by maintenance RITUXAN® (Rituximab).
REVLIMID® (Lenalidomide) is an oral immunomodulatory agent (IMiD) with activity in lymphoid malignancies, primarily through immune modulation (repair T-cell immune synapse dysfunction and Natural Killer cell/T-cell effector augmentation). It additionally has antiproliferative effects. Chemo-free combination immunotherapy with REVLIMID® and RITUXAN® or the R2 regimen, has shown promising activity in phase II studies.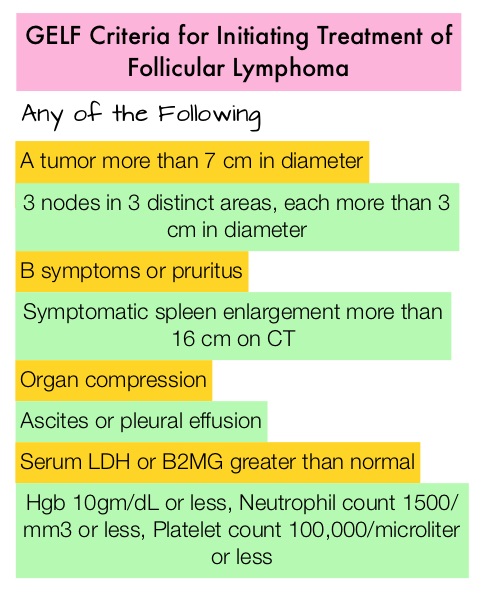
RELEVANCE is a global, randomized, open-label, phase III study, conducted in partnership with the Lymphoma Academic Research Organisation (LYSARC). This study evaluated the investigational regimen of REVLIMID® plus RITUXAN® (R2), followed by RITUXAN® maintenance, compared to the standard of care treatment of RITUXAN® plus chemotherapy, followed by RITUXAN® maintenance, in patients with previously untreated Follicular Lymphoma.
In this study, 1030 patients with treatment naïve, advanced Follicular Lymphoma, were randomized to R2 regimen (N=513) and R-Chemo regimen (N=517). Patients had Grade 1-3a Follicular Lymphoma, requiring therapy according to GELF criteria. Patients in the R2 group received REVLIMID® 20 mg orally daily on Days 2 thru 22 every 28 days for 6-12 cycles and continued responders received REVLIMID® 10 mg orally daily on Days 2 thru 22 every 28 days, for a total of 18 cycles. RITUXAN® was administered at 375 mg/m2 IV on Days 1, 8, 15, and 22 of cycle 1 and Day 1 of cycles 2 thru 6, and then continued in responders for 12 additional cycles every 8 weeks. R-Chemo group received investigators choice of standard R-CHOP (72%), R-Bendamustine (23%) or R-CVP (5%), and responding patients continued with RITUXAN® 375 mg/m2 IV every 8 weeks, for 12 cycles. The median age of the patients was 59 years. The co-Primary endpoints were Complete Response/unconfirmed Complete Response at 120 weeks and Progression Free Survival (PFS) during the preplanned analysis.
At a median follow up of 37.9 months, PFS was similar in both treatment groups and the 3-year PFS rate was 77% in the R2 group compared with 78% for the R-Chemo group (HR=1.10; P=0.48). The Complete Response/unconfirmed Complete Response at 120 weeks were 48% in the R2 group and 53% in the R-chemo group and this was also not statistically significant (P=0.13). Preliminary Overall Survival outcomes (Secondary endpoint) showed a 3-year survival rate of 94% in both treatment groups. Adverse events were different in the two treatment groups, with a higher incidence of neutropenia and febrile neutropenia in the R-Chemo group, and higher incidence of cutaneous events in the R2 group.
It was concluded that in this first randomized phase III comparison of a chemo-free regimen (R2) with standard R-Chemo, in previously untreated Follicular Lymphoma, a combination of REVLIMID® and RITUXAN® (R2) showed similar efficacy, with a more favorable safety profile, making it a potential chemo-free, firstline option, for patients with Follicular Lymphoma. RELEVANCE: Phase III randomized study of lenalidomide plus rituximab (R2) versus chemotherapy plus rituximab, followed by rituximab maintenance, in patients with previously untreated follicular lymphoma. Fowler NH, Morschhauser F, Feugier P, et al. J Clin Oncol 36, 2018 (suppl; abstr 7500)

