SUMMARY: Kidney cancer is among the 10 most common cancers in both men and women and the American Cancer Society estimates that approximately 64,000 new cases of kidney cancer will be diagnosed for 2014 in the United States and about 13,860 individuals will die from this disease. Renal cell carcinoma is a highly vascular tumor. Vascular Endothelial Growth Factor (VEGF), PDGF (Platelet Derived Growth Factor) and FGF (Fibroblast Growth Factor) are the major growth factors involved in angiogenesis, with VEGF playing the most important role, regulating vascular permeability. These ligands (VEGF, PDGF and FGF) bind to their respective receptors which exhibit tyrosine kinase activity and are able to activate the signaling pathways down stream. The study of Von Hippel-Lindau (VHL) syndrome and the cloning of VHL gene (a tumor suppressor gene) led to the understanding of the molecular basis and biology of the most common type of sporadic Renal Cell Carcinoma (Clear Cell Carcinoma). All patients with VHL syndrome have a germ line inactivating mutation of one VHL allele and inactivation of the second allele is the triggering event for the formation of Clear Cell Carcinomas of the kidney, as well as other tumors including endocrine Pancreatic tumors, Papillary cystadenomas of the pancreas, adnexal organs, epididymis, endolymphatic system of the inner ear, Hemangioblastoma of the CNS and retina and Pheochromocytomas. It appears that close to 60% of the patients with non-hereditary (sporadic) clear cell carcinoma of the kidney have loss of function of the VHL gene.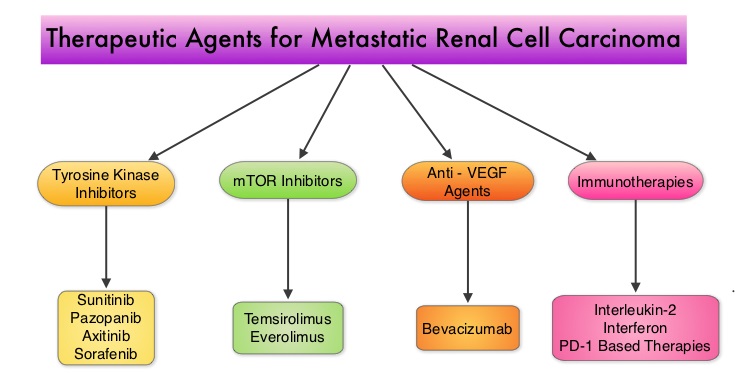 The understanding of the biology of clear cell carcinoma of the kidney has led to the development of multitargeted Tyrosine Kinase Inhibitors, which are small molecules that simultaneously target the tumor cell wall, vascular endothelial cell wall as well as the pericyte/fibroblast/vascular/ smooth vessel cell wall. The end result is decreased angiogenesis, cell proliferation and cell survival of both endothelial cells as well as tumor cells. There are four classes of agents presently available for the treatment of metastatic Renal Cell carcinoma. They include Immunotherapies, Anti-VEGF agents, Tyrosine Kinase Inhibitors (TKI’s) and mTOR (Mammalian Target of Rapamycin) inhibitors. The choice of First Line therapies for metastatic Renal Cell Carcinoma is based on MSKCC (Memorial Sloan Kettering Cancer Center) risk classification and the agents include, Temsirolimus (TORISEL®) for poor risk patients and TKI’s as well as Immunotherapy with or without anti-VEGF agents for good and intermediate risk patients.
The understanding of the biology of clear cell carcinoma of the kidney has led to the development of multitargeted Tyrosine Kinase Inhibitors, which are small molecules that simultaneously target the tumor cell wall, vascular endothelial cell wall as well as the pericyte/fibroblast/vascular/ smooth vessel cell wall. The end result is decreased angiogenesis, cell proliferation and cell survival of both endothelial cells as well as tumor cells. There are four classes of agents presently available for the treatment of metastatic Renal Cell carcinoma. They include Immunotherapies, Anti-VEGF agents, Tyrosine Kinase Inhibitors (TKI’s) and mTOR (Mammalian Target of Rapamycin) inhibitors. The choice of First Line therapies for metastatic Renal Cell Carcinoma is based on MSKCC (Memorial Sloan Kettering Cancer Center) risk classification and the agents include, Temsirolimus (TORISEL®) for poor risk patients and TKI’s as well as Immunotherapy with or without anti-VEGF agents for good and intermediate risk patients. 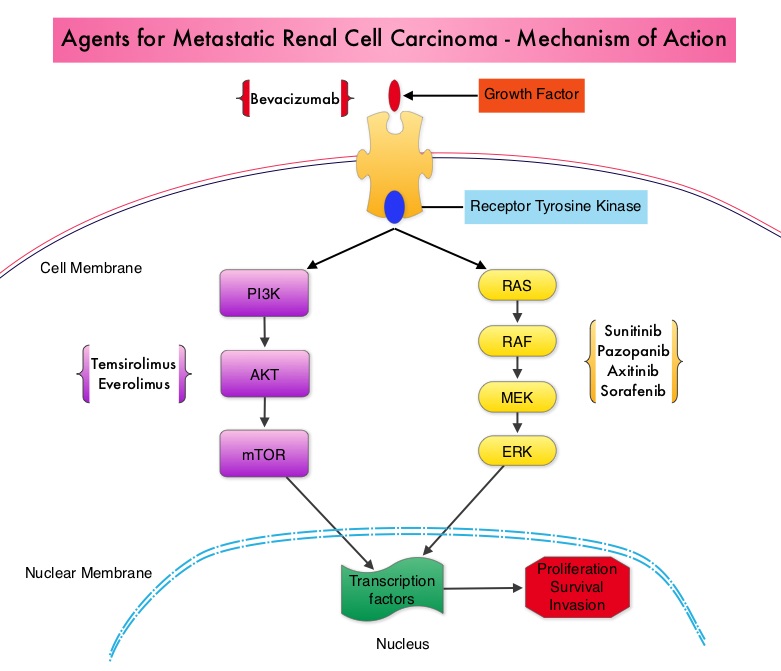 The TKI’s of choice however for good to intermediate risk mRCC patients with clear cell histology are, either SUTENT® (Sunitinib) or VOTRIENT® (Pazopanib). Even though both agents have similar efficacy, it remained unclear if one agent was better tolerated than the other. To address this, the authors evaluated patient preference for VOTRIENT® or SUTENT® in a double blind, phase III cross over study. The primary end point of this study was patient preference for a specific treatment, as assessed by a questionnaire at the end of the two treatment periods. Other endpoints included reasons for preference, physician preference, safety, and HRQoL (Health Related Quality of Life). Randomly assigned patients with metastatic Renal Cell Carcinoma (mRCC) with clear cell histology (N=168), received VOTRIENT® 800 mg per day for 10 weeks followed by a 2 week washout period and then SUTENT® 50 mg per day (4 weeks on, 2 weeks off, 4 weeks on) for 10 weeks (N=86), or the reverse sequence (N=82). One hundred and fourteen (N=114) patients met the prespecified modified intent-to-treat criteria for the primary analysis and the criteria included exposure to both of the treatments, no disease progression before cross over and completion of the preference questionnaire. When outcomes were evaluated, 70% of the patients preferred VOTRIENT®, 22% preferred SUTENT® and 8% had no preference (P<0.001).
The TKI’s of choice however for good to intermediate risk mRCC patients with clear cell histology are, either SUTENT® (Sunitinib) or VOTRIENT® (Pazopanib). Even though both agents have similar efficacy, it remained unclear if one agent was better tolerated than the other. To address this, the authors evaluated patient preference for VOTRIENT® or SUTENT® in a double blind, phase III cross over study. The primary end point of this study was patient preference for a specific treatment, as assessed by a questionnaire at the end of the two treatment periods. Other endpoints included reasons for preference, physician preference, safety, and HRQoL (Health Related Quality of Life). Randomly assigned patients with metastatic Renal Cell Carcinoma (mRCC) with clear cell histology (N=168), received VOTRIENT® 800 mg per day for 10 weeks followed by a 2 week washout period and then SUTENT® 50 mg per day (4 weeks on, 2 weeks off, 4 weeks on) for 10 weeks (N=86), or the reverse sequence (N=82). One hundred and fourteen (N=114) patients met the prespecified modified intent-to-treat criteria for the primary analysis and the criteria included exposure to both of the treatments, no disease progression before cross over and completion of the preference questionnaire. When outcomes were evaluated, 70% of the patients preferred VOTRIENT®, 22% preferred SUTENT® and 8% had no preference (P<0.001). 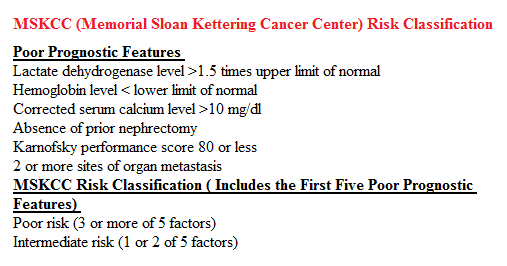 The main reasons for preferring VOTRIENT® were less fatigue and better overall quality of life whereas those who preferred SUTENT® cited less diarrhea as the main reason. Physician preference was also taken into consideration in this study, as physicians are able to better assess efficacy and asymptomatic toxicities that are clinically relevant. VOTRIENT® was preferred by 61% of the physicians, 22% preferred SUTENT® and 17% had no preference. VOTRIENT® was also superior to SUTENT® with regards to HRQoL measures that evaluated fatigue, hand/foot soreness and mouth/throat soreness. The authors concluded that this innovative cross-over trial demonstrated that significantly more patients preferred VOTRIENT® over SUTENT®, based on lower rates of adverse events and better Health-Related Quality of Life. Escudier B, Porta C, Bono P, et al. J Clin Oncol 2014;32:1412-1418
The main reasons for preferring VOTRIENT® were less fatigue and better overall quality of life whereas those who preferred SUTENT® cited less diarrhea as the main reason. Physician preference was also taken into consideration in this study, as physicians are able to better assess efficacy and asymptomatic toxicities that are clinically relevant. VOTRIENT® was preferred by 61% of the physicians, 22% preferred SUTENT® and 17% had no preference. VOTRIENT® was also superior to SUTENT® with regards to HRQoL measures that evaluated fatigue, hand/foot soreness and mouth/throat soreness. The authors concluded that this innovative cross-over trial demonstrated that significantly more patients preferred VOTRIENT® over SUTENT®, based on lower rates of adverse events and better Health-Related Quality of Life. Escudier B, Porta C, Bono P, et al. J Clin Oncol 2014;32:1412-1418

