SUMMARY: Prostate cancer is the most common cancer in American men with the exclusion of skin cancer, and 1 in 9 men will be diagnosed with prostate cancer during their lifetime. It is estimated that in the United States, about 191,930 new cases of prostate cancer will be diagnosed in 2020 and 33,330 men will die of the disease.
The major source of PSA (Prostate Specific Antigen) is the prostate gland and the PSA levels are therefore undetectable within 6 weeks after Radical Prostatectomy. Similarly, following Radiation Therapy, there is a gradual decline in PSA before reaching a post treatment nadir. A detectable PSA level after Radical Prostatectomy, or a rising PSA level following Radiation Therapy, is considered PSA failure or biochemical recurrence. Approximately 35% of the patients with prostate cancer will experience PSA only relapse within 10 years of their primary treatment and a third of these patients will develop documented metastatic disease within 8 years following PSA only relapse.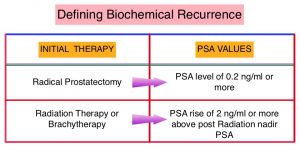
Rising PSA is therefore a sign of recurrent disease and identifying the site of recurrence can be of immense value for the clinician and can help determine the best course of therapy. The diagnostic accuracy of standard imaging tests, for the identification of sites of recurrence in patients with biochemical recurrence, is low. Almost 90% of the standard imaging tests such as CT/MRI and Bone Scan may be negative. More accurate non-invasive imaging techniques for the detection of recurrent tumor is therefore an unmet need. Prostascint, a Single Photon Emission Computerized Tomography (SPECT) radiopharmaceutical agent, was approved in 1999 for the diagnostic imaging of post-prostatectomy patients with a rising PSA. PET (Positron Emission Tomography) scans have largely superseded this study. FluDeoxyGlucose F18 (FDG), a glucose analogue is the most widely used PET radiotracer, but is not generally used as an imaging agent in prostate cancer. This is because good and reliable quality images are not feasible due to indolent growth of prostate cancers and the high urinary excretion of FDG. The other PET radiotracer that is available, Choline C11, has been shown to improve cancer detection in men with biochemical recurrent prostate cancer, but this agent has a short half life of 20 minutes, requires greater patient preparation including 6 hours of fasting prior to administration of Choline C11, delivers higher radiation dose to patients and image quality is poor. The FDA in 2016 approved AXUMIN® (Fluciclovine F18), a novel molecular radiopharmaceutical diagnostic agent, for PET imaging in men with suspected prostate cancer recurrence, based on elevated PSA levels, following prior treatment. This study however is less likely to be positive with PSA less than 1 ng/mL, unless the doubling time is rapid. There is also higher false positive rate within the intact or treated prostate gland, and uptake may be absent in densely sclerotic lesions. Current imaging modalities are therefore inadequate for localizing and characterizing occult disease in men with biochemically recurrent prostate cancer.
F-18 DCFPyL is a novel PET imaging agent that binds selectively with high affinity to Prostate-Specific Membrane Antigen (PSMA), which is overexpressed in prostate cancer cells. CONDOR is a prospective, multicenter, randomized, Phase III trial, conducted to evaluate the diagnostic performance of PET/CT imaging with F-18 DCFPyL, a radiopharmaceutical targeting the extracellular domain of PSMA. This study enrolled 208 men at 14 sites in the US and Canada, with a rising PSA level after definitive therapy and negative or equivocal standard-of-care imaging (eg, CT, MRI, bone scintigraphy). PET/CT imaging was performed 1-2 hours following administration of a single dose of F-18 DCFPyL. The median age was 68 yrs and the median time from diagnosis was 71 months. Approximately 50% of all patients had undergone Radical Prostatectomy, 35% underwent Radical Prostatectomy and Radiation Therapy, 15% had only received RadioTherapy, and 28% received at least one systemic therapy for their prostate cancer. Approximately 74% of patients had a total Gleason score below 8. All enrolled patients had biochemically recurrent metastatic Castration-Resistant Prostate Cancer, and a PSA of at least 0.2 ng/mL following radical prostatectomy, or at least 2 ng/mL over the nadir following prior Radiation Therapy, Cryotherapy or systemic therapy. The median PSA was 0.8 ng/mL, (PSA level at which most decisions about subsequent salvage focal or systemic therapies are made) and 31% of patients had a PSA of at least 2.0 ng/mL. All enrolled patients had no previous radiologic findings. The Primary endpoint was Correct Localization Rate of occult disease, as determined by three independent reviewers, and the Secondary endpoint was the impact of F-18 DCFPyL PET/CT imaging results on management of enrolled patients in this study.
The study met its Primary endpoint and the Correct Localization Rate of occult disease or the Positive Predictive Value ranged from 84.8% to 87% for the three independent reviewers. The Correct Localization Rate of occult disease was maintained regardless of PSA values and the F-18 DCFPyL PET/CT imaging detected disease even at the lowest of PSA values. Regarding the Secondary endpoint of impact of F-18 DCFPyL PET/CT imaging on treatment, 64% of patients had a change in management due to findings noted on the imaging study, of which 78% were attributable to positive findings on the imaging study, and 21.4% to negative findings on F-18 DCFPyL PET/CT imaging study. Specific changes in the treatment management included change in the goal of patients disease management from a noncurative approach to a curative salvage local therapy in 21% of patients, 28% changed from receiving salvage local therapy to systemic therapy or added systemic therapy, 23.9% changed from observation status to initiation of therapy and 4.4% changed from planned treatment to observation alone.
It was concluded that PSMA-targeted F-18 DCFPyL PET/CT imaging detected and localized occult disease in most men with biochemical recurrence, presenting with negative or equivocal findings on conventional imaging. Further, F-18 DCFPyL PET/CT imaging provided actionable information that led to change in treatment plans for the majority of patients, thus providing evidence that PSMA PET imaging may be valuable in men with recurrent or suspected metastatic prostate cancer.
Impact of PSMA-targeted imaging with 18F-DCFPyL-PET/CT on clinical management of patients (pts) with biochemically recurrent (BCR) prostate cancer (PCa): Results from a phase III, prospective, multicenter study (CONDOR). Morris MJ, Carroll PR, Saperstein L, et al. DOI: 10.1200/JCO.2020.38.15_suppl.5501 Journal of Clinical Oncology 38, no. 15_suppl (May 20, 2020) 5501-5501.

