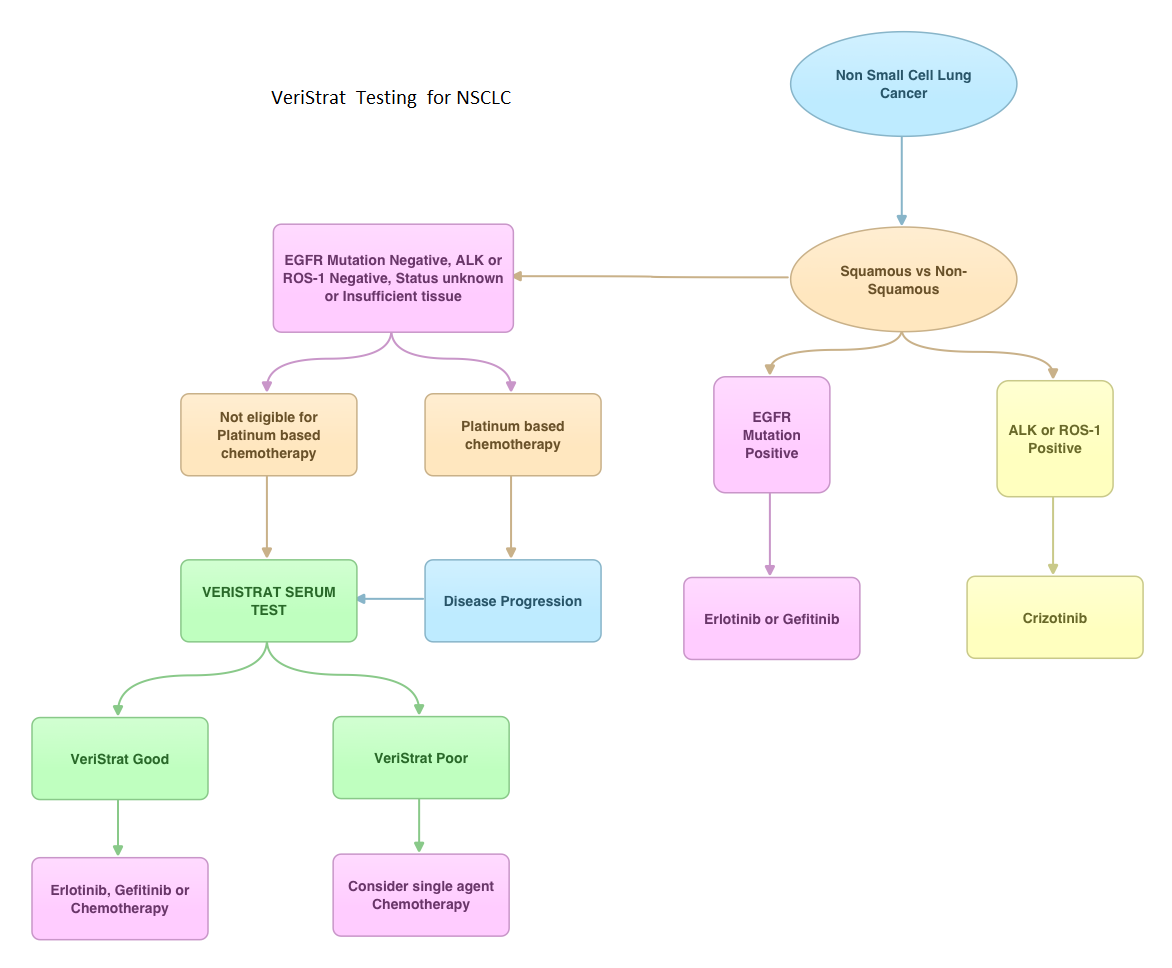
SUMMARY: VeriStrat ® is a clinically validated serum/plasma-based assay, for patients with advanced Non Small Cell Lung Cancer (NSCLC). VeriStrat® is a serum test of prognostic and predictive value that classifies patients as VeriStrat-Good (VS-G) or VeriStrat-Poor (VS-P) based on eight mass spectral peaks or proteomic patterns of the patients serum. Proteomics is the large-scale study of protein structure and functions. VeriStrat® testing is protein based and therefore has no correlation with known genomic biomarkers. It is well established that EGFR-TKIs (Epidermal Growth Factor Receptor-Tyrosine Kinase Inhibitors) are more effective in NSCLC patients with EGFR activating mutations. PROSE is a multicenter, double blind, randomized, VeriStrat® stratified, phase III study. In this trial, over 90% of the patients had no EGFR mutations (EGFR-Wild Type). Two hundred and eighty five (285) patients with advanced NSCLC who had first line treatment regimen with platinum-based therapy were randomly assigned to receive second line chemotherapy (CT) with single agent ALIMTA® (Pemetrexed) or TAXOTERE® (Docetaxel), at standard doses (N=129) or TARCEVA® (Erlotinib) 150 mg po qd (N=134). Patients and study investigators were blinded to the patients VeriStrat® status. Patients were classified as VeriStrat-Good or VeriStrat-Poor based on the VeriStrat® results. Patients in the treatment groups were stratified by age, gender, tumor histology, ECOG-PS and smoking history. Crossover was permitted upon disease progression. The primary objective of the study was to demonstrate differential treatment benefit between TARCEVA® and CT with regards to Overall Survival (OS). Median overall survival (OS) was 9 months for the patients in the CT group and 7.7 months for TARCEVA® group and this was not statistically significant (P=0.3). However when evaluated by VeriStrat® status, CT was beneficial for the VeriStrat-Poor patients compared to TARCEVA®, with significantly better median OS (6.3 vs 3 months, P=0.02). Age, gender, histology (squamous vs non-squamous) and smoking history had no impact on the overall survival. The authors concluded that patients classified as VeriStrat-Poor have better survival with CT than TARCEVA®, whereas patients classified as VeriStrat-Good have similar survival with TARCEVA® and CT. VeriStrat® testing therefore, can help physicians choose between TARCEVA® and CT, for their patients with advanced NSCLC. This test helps physicians identify patients who are likely to have good or poor outcomes after treatment with EGFR inhibitors and thereby can provide valuable insight into whether CT or targeted therapy with TARCEVA®, a EGFR-TKI, is appropriate for their patients with advanced NSCLC, in the second line setting. This information is especially important for patients without an EGFR mutation or for those, whose EGFR mutation status is unknown. Sorlini C, Barni S, Petrelli F, et al. J Clin Oncol 29: 2011 (suppl; abstr TPS214)

