SUMMARY: Multiple Myeloma is a clonal disorder of plasma cells in the bone marrow and the American Cancer Society estimates that in the United States, close to 27,000 new cases will be diagnosed in 2015 and 11,240 will die of the disease. REVLIMID® (Lenalidomide) given along with weekly Dexamethasone, was associated with significantly improved Progression Free Survival (PFS) when administered until disease progression, in patients with newly diagnosed Multiple Myeloma. This combination of REVLIMID® and weekly Dexamethasone is considered a reference regimen (Control arm) for both newly diagnosed and relapsed Multiple Myeloma. Elotuzumab (HuLuc63) is a monoclonal antibody that binds to the Signal Lymphocyte Activation Molecule – SLAMF7 protein (CS1, CD319), which is highly expressed on Myeloma cells and also expressed on Natural Killer (NK) lymphocytes in the immune system. 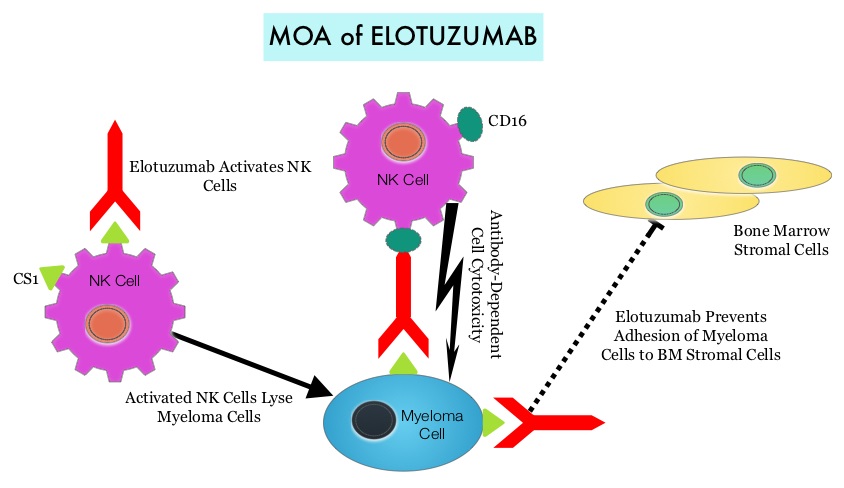 By virtue of its dual mechanism of action, it targets and destroys Myeloma cells and also enhances the activation of Natural Killer cells. Previously published phase Ib/II study, has shown encouraging activity, when Elotuzumab was combined with REVLIMID® and Dexamethasone, in patients with Relapsed/Refractory Multiple Myeloma (RRMM). ELOQUENT-2 is an open-label phase III trial in which 646 patients with Relapsed/Refractory Multiple Myeloma were randomized in a 1:1 ratio to receive Elotuzumab in combination with REVLIMID® and Dexamethasone (N=321) or REVLIMID® and Dexamethasone alone (N=325). Enrolled patients had 1–3 prior therapies and were not REVLIMID® refractory. Prior therapies included VELCADE® (Bortezomib), THALOMID® (Thalidomide) and REVLIMID®. Approximately 35% of the enrollees were refractory to the last therapy, 32% had del(17p) and 9% had t(4;14). The median age was 66 years. Elotuzumab was administered at 10 mg/kg IV weekly for the first two cycles and then once every 2 weeks thereafter. REVLIMID® was given at 25 mg orally on days 1 thru 21 of each cycle along with Dexamethasone 40 mg weekly. In the Elotuzumab group, Dexamethasone was dosed at 28 mg orally plus 8 mg IV on the weeks when Elotuzumab was administered. The cycle duration was 28 days. Treatment was administered until disease progression or unacceptable toxicity. Primary endpoints were Progression Free Survival (PFS) and Overall Response Rate (ORR). At a median follow up of 24 months, PFS in the Elotuzumab group was 19.4 months compared to 14.9 months in the REVLIMID®/Dexamethasone alone group (HR=0.70; P=0.0004). The 1-year PFS for the Elotuzumab versus control group was 68% vs 57% respectively and the 2-year PFS was 41% vs 27%. This benefit was seen across all subgroups including those with unfavorable cytogenetics. The ORR was 79% in the Elotuzumab group and 66% in the control group. (P = 0.0002). At the time of this interim analysis, more patients in the Elotuzumab group remained on therapy (35%) compared to the control group (21%) and treatment discontinuation was mainly for disease progression. Grade 3–4 toxicities occurred in 15% or more patients in the Elotuzumab group and included neutropenia and anemia. The authors concluded that Elotuzumab with its novel immunotherapeutic mechanism of action, when added to REVLIMID® and Dexamethasone, reduced the risk of disease progression by 30% in patients with Relapsed/Refractory MultipleMyeloma, and this was accomplished with manageable toxicities. Patients in this study are being followed up for long term outcomes including Overall Survival. Lonial S, Dimopoulos MA, Palumbo A, et al. ELOQUENT-2: A phase III, randomized, open-label study of lenalidomide (Len)/dexamethasone (dex) with/without elotuzumab (Elo) in patients (pts) with relapsed/refractory multiple myeloma (RRMM). J Clin Oncol. 2015;(suppl; abstr 8508).</s
By virtue of its dual mechanism of action, it targets and destroys Myeloma cells and also enhances the activation of Natural Killer cells. Previously published phase Ib/II study, has shown encouraging activity, when Elotuzumab was combined with REVLIMID® and Dexamethasone, in patients with Relapsed/Refractory Multiple Myeloma (RRMM). ELOQUENT-2 is an open-label phase III trial in which 646 patients with Relapsed/Refractory Multiple Myeloma were randomized in a 1:1 ratio to receive Elotuzumab in combination with REVLIMID® and Dexamethasone (N=321) or REVLIMID® and Dexamethasone alone (N=325). Enrolled patients had 1–3 prior therapies and were not REVLIMID® refractory. Prior therapies included VELCADE® (Bortezomib), THALOMID® (Thalidomide) and REVLIMID®. Approximately 35% of the enrollees were refractory to the last therapy, 32% had del(17p) and 9% had t(4;14). The median age was 66 years. Elotuzumab was administered at 10 mg/kg IV weekly for the first two cycles and then once every 2 weeks thereafter. REVLIMID® was given at 25 mg orally on days 1 thru 21 of each cycle along with Dexamethasone 40 mg weekly. In the Elotuzumab group, Dexamethasone was dosed at 28 mg orally plus 8 mg IV on the weeks when Elotuzumab was administered. The cycle duration was 28 days. Treatment was administered until disease progression or unacceptable toxicity. Primary endpoints were Progression Free Survival (PFS) and Overall Response Rate (ORR). At a median follow up of 24 months, PFS in the Elotuzumab group was 19.4 months compared to 14.9 months in the REVLIMID®/Dexamethasone alone group (HR=0.70; P=0.0004). The 1-year PFS for the Elotuzumab versus control group was 68% vs 57% respectively and the 2-year PFS was 41% vs 27%. This benefit was seen across all subgroups including those with unfavorable cytogenetics. The ORR was 79% in the Elotuzumab group and 66% in the control group. (P = 0.0002). At the time of this interim analysis, more patients in the Elotuzumab group remained on therapy (35%) compared to the control group (21%) and treatment discontinuation was mainly for disease progression. Grade 3–4 toxicities occurred in 15% or more patients in the Elotuzumab group and included neutropenia and anemia. The authors concluded that Elotuzumab with its novel immunotherapeutic mechanism of action, when added to REVLIMID® and Dexamethasone, reduced the risk of disease progression by 30% in patients with Relapsed/Refractory MultipleMyeloma, and this was accomplished with manageable toxicities. Patients in this study are being followed up for long term outcomes including Overall Survival. Lonial S, Dimopoulos MA, Palumbo A, et al. ELOQUENT-2: A phase III, randomized, open-label study of lenalidomide (Len)/dexamethasone (dex) with/without elotuzumab (Elo) in patients (pts) with relapsed/refractory multiple myeloma (RRMM). J Clin Oncol. 2015;(suppl; abstr 8508).</s

