SUMMARY: It is estimated that in the United States, approximately 21,750 women will be diagnosed with ovarian cancer in 2020 and 13,940 women will die of the disease. Ovarian cancer ranks fifth in cancer deaths among women, and accounts for more deaths than any other cancer of the female reproductive system. Approximately 75% of the ovarian cancer patients are diagnosed with advanced disease. Patients with newly diagnosed advanced ovarian cancer are often treated with platinum based chemotherapy following primary surgical cytoreduction. Approximately 70% of these patients will relapse within the subsequent 3 years and are incurable, with a 5 year Overall Survival rate of about 20-30%.
DNA damage is a common occurrence in daily life by UV light, ionizing radiation, replication errors, chemical agents, etc. This can result in single and double strand breaks in the DNA structure which must be repaired for cell survival. The two vital pathways for DNA repair in a normal cell are BRCA1/BRCA2 and PARP. BRCA1 and BRCA2 are tumor suppressor genes and they recognize and repair double strand DNA breaks via Homologous Recombination (HR) pathway. Homologous Recombination is a DNA repair pathway utilized by cells to accurately repair DNA double-stranded breaks during the S and G2 phases of the cell cycle, and thereby maintain genomic integrity.
Homologous Recombination Deficiency (HRD) is noted following mutation of genes involved in HR repair pathway. At least 15 genes are involved in the Homologous Recombination Repair (HRR) pathway including BRCA1 and BRCA2 genes. The BRCA1 gene is located on the long (q) arm of chromosome 17 whereas BRCA2 is located on the long arm of chromosome 13, and they regulate cell growth and prevent abnormal cell division and development of malignancy. Mutations in BRCA1 and BRCA2 account for about 20-25% of hereditary breast cancers 15% of ovarian cancers, in addition to other cancers such as Colon and Prostate. BRCA mutations can either be inherited (Germline) and present in all individual cells or can be acquired and occur exclusively in the tumor cells (Somatic).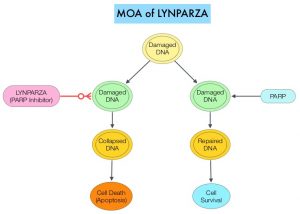
The PARP (Poly ADP Ribose Polymerase) family of enzymes include PARP1 and PARP2, and is a related enzymatic pathway that repairs single strand breaks in DNA. In a BRCA mutant, the cancer cell relies solely on PARP pathway for DNA repair to survive. LYNPARZA® is a PARP inhibitor that traps PARP onto DNA at sites of single-strand breaks, preventing their repair and generating double-strand breaks that cannot be repaired accurately in tumors harboring defects in Homologous Recombination Repair pathway genes, such as BRCA1 or BRCA2 mutations, leading to cumulative DNA damage and tumor cell death.
Previously published studies demonstrated a durable response to LYNPARZA® administered as treatment (rather than maintenance), in women with heavily pretreated relapsed ovarian cancer and a germline BRCA mutation, with an Objective Response Rate (ORR) of 42% in the subgroup of patients with platinum-sensitive disease, who had received at least 3 prior chemotherapy regimens. Single-agent nonplatinum chemotherapy is often used in heavily pretreated women with relapsed ovarian cancer. The authors conducted this study to evaluate whether LYNPARZA® monotherapy improves outcomes, compared with physician’s choice single-agent nonplatinum chemotherapy, in patients with platinum-sensitive relapsed ovarian cancer and a germline BRCA mutation, who have received at least 2 prior lines of platinum-based chemotherapy.
SOLO3 is an International, randomized, controlled, open label Phase III trial, that compared LYNPARZA® with non-platinum chemotherapy, in patients with platinum sensitive, relapsed ovarian cancer, and a germline BRCA1/2 mutation. This study included 266 patients who were randomly assigned 2:1 to LYNPARZA® 300 mg orally given twice a day (N=178) or physician’s choice of single-agent chemotherapy (N=88), which could be either Pegylated Liposomal Doxorubicin (PLD) 50 mg/m2 IV on day 1 every 4 weeks, Paclitaxel 80 mg/m2 IV on days 1, 8, 15, and 22 every 4 weeks, Gemcitabine 1000 mg/m2 IV on days 1, 8, and 15 every 4 weeks or Topotecan 4 mg/m2 IV on days 1, 8, and 15 every 4 weeks. Eligible patients had relapsed high-grade serous or high-grade endometrioid ovarian cancer, primary peritoneal cancer, and/or fallopian tube cancer, with at least 1 measurable and/or nonmeasurable lesion, that could be accurately assessed at baseline, by CT or MRI, and was suitable for repeated evaluation. Patients had received at least 2 prior lines of platinum-based chemotherapy for ovarian cancer and were platinum sensitive (progression more than 6 months after the end of the last platinum-based regimen). Treatment groups were well balanced and the median patient age was 59 years. The Primary end point was Objective Response Rate (ORR) in those with measurable disease, as assessed by Blinded Independent Central Review (BICR). The key Secondary end point was Progression Free Survival (PFS) assessed by BICR in the intent-to-treat population.
It was noted that ORR was significantly higher in the LYNPARZA® group than in the chemotherapy group (72.2% versus 51.4%; Odds Ratio=2.53; P=0.002), suggesting a 2.53 times higher likelihood of responding to LYNPARZA®, than to chemotherapy. In the subgroup who had received 2 prior lines of treatment, the ORR with LYNPARZA® was 84.6% and 61.5% with chemotherapy (Odds Ratio= 3.44), suggesting a 3.44 times higher likelihood of responding to LYNPARZA®, than to chemotherapy. The median time to onset of response was 2 months with LYNPARZA®, versus 3.5 months with chemotherapy, and the median Duration of Response was 9.4 months and 10.2 months respectively. The PFS also significantly favored LYNPARZA® versus chemotherapy (13.4 versus 9.2 months; HR=0.62; P=0.013). Adverse events were consistent with the established safety profiles of LYNPARZA® and chemotherapy. The most common Grade 3 or more adverse events were anemia in the LYNPARZA® group and PPE (Palmar-Plantar Erythrodysesthesia) and neutropenia in the chemotherapy group.
It was concluded that treatment with LYNPARZA® resulted in statistically significant and clinically relevant improvements in Objective Response Rate and Progression Free Survival, compared with nonplatinum chemotherapy, in patients with germline BRCA-mutated, platinum-sensitive, relapsed ovarian cancer, who had received at least 2 prior lines of platinum-based chemotherapy. This chemotherapy-free treatment option will be welcome news for patients with germline BRCA-mutated advanced ovarian cancer.
Olaparib Versus Nonplatinum Chemotherapy in Patients With Platinum-Sensitive Relapsed Ovarian Cancer and a Germline BRCA1/2 Mutation (SOLO3): A Randomized Phase III Trial. Penson RT, Valencia RV, Cibula D, et al. J Clin Oncol. 2020;38:1164-1174.

