SUMMARY: Prostate cancer is the most common cancer in American men with the exclusion of skin cancer, and 1 in 9 men will be diagnosed with Prostate cancer during their lifetime. It is estimated that in the United States, about 174,650 new cases of Prostate cancer will be diagnosed in 2019 and 31,620 men will die of the disease. The development and progression of Prostate cancer is driven by androgens. Androgen Deprivation Therapy (ADT) or testosterone suppression has therefore been the cornerstone of treatment of advanced Prostate cancer and is the first treatment intervention. Androgen Deprivation Therapies have included bilateral orchiectomy or Gonadotropin Releasing Hormone (GnRH) analogues, with or without first generation Androgen Receptor (AR) inhibitors such as CASODEX® (Bicalutamide), NILANDRON® (Nilutamide) and EULEXIN® (Flutamide) or with second-generation, anti-androgen agents, which include, ZYTIGA® (Abiraterone), XTANDI® (Enzalutamide) and ERLEADA® (Apalutamide). ZYTIGA® inhibits CYP17A1 enzyme and depletes adrenal and intratumoral androgens, thereby impairing AR signaling. XTANDI® and ERLEADA® compete with Testosterone and Dihydrotestosterone and avidly binds to the Androgen Receptor, thereby inhibiting AR signaling, and in addition inhibits translocation of the AR into the nucleus and thus inhibits the transcriptional activities of the AR.
Approximately 10-20% of patients with advanced Prostate cancer will progress to Castration Resistant Prostate Cancer (CRPC) within five years during ADT, and over 80% of these patients will have metastatic disease at the time of CRPC diagnosis. Among those patients without metastases at CRPC diagnosis, 33% are likely to develop metastases within two years. The estimated mean survival of patients with CRPC is 9-36 months and there is therefore an unmet need for new effective therapies.
The malignant transformation of prostatic epithelial cell as well as the development of CRPC has been attributed to deleterious alterations in a variety of genes including loss-of-function alterations in Homologous Recombination repair genes such as BRCA1, BRCA2 and ATM. Homologous Recombination (HR) is a type of genetic recombination, and this DNA repair pathway is used by cells to accurately repair DNA double-stranded breaks (DSBs) and thereby maintain genomic integrity. Homologous Recombination Deficiency (HRD) is noted following mutation of genes involved in HR repair pathway. At least 15 genes are involved in the Homologous Recombination Repair (HRR) pathway including BRCA1 and BRCA2 genes. BRCA1 and BRCA2 are tumor suppressor genes and functional BRCA proteins repair damaged DNA, and play an important role in maintaining cellular genetic integrity. They regulate cell growth and prevent abnormal cell division and development of malignancy. Mutations in BRCA1 and BRCA2 account for about 20-25% of hereditary breast cancers and about 5-10% of all breast cancers. They also account for 15% of ovarian cancers, in addition to other cancers such as Colon and Prostate. BRCA mutations can either be inherited (Germline) and present in all individual cells or can be acquired and occur exclusively in the tumor cells (Somatic). Somatic mutations account for a significant portion of overall BRCA1 and BRCA2 aberrations. Loss of BRCA function due to frequent somatic aberrations likely deregulates Homologous Recombination (HR) pathway and increases sensitivity to platinum drugs. Majority of the individuals with Germline BRCA mutations (gBRCA) are positive for HR deficiency.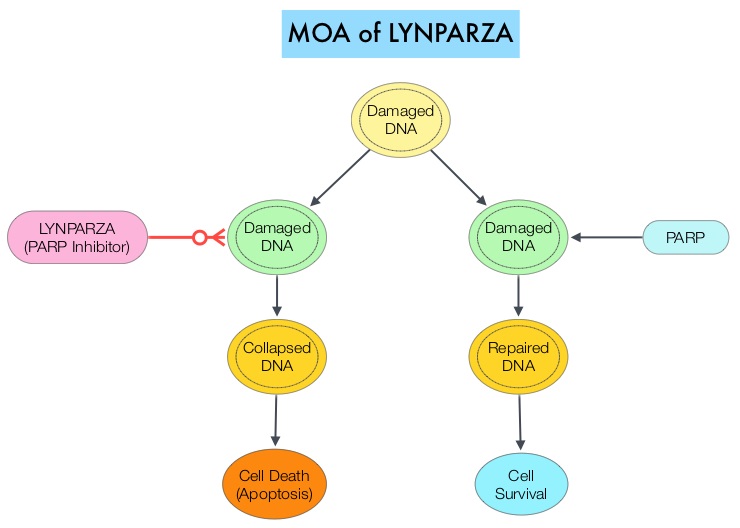
The PARP (Poly ADP Ribose Polymerase) family of enzymes which include PARP1 and PARP2 repair damaged DNA. PARP inhibitors inhibit the PARP protein in cancer cells and kill tumors defective in the BRCA1 or BRCA2 genes through the concept of synthetic lethality. LYNPARZA® (Olaparib) is a first-in-class PARP inhibitor and blocks DNA damage response in tumors harboring a deficiency in Homologous Recombination Repair, as is noted in those with mutations such as BRCA1 and/or BRCA2. LYNPARZA® showed promising results in a Phase II trial (TOPARP), when given as monotherapy, in patients with BRCA1/2 or ATM gene-mutated mCRPC, who had received a prior Taxane-based chemotherapy, and at least one newer hormonal agent (ZYTIGA® or XTANDI®).
PROfound is a prospective, multicentre, randomized, open-label, Phase III trial in which the efficacy and safety of LYNPARZA® was compared with physician’s choice of either XTANDI® or ZYTIGA® in two groups of patients with mCRPC, who had progressed on prior treatment with new hormonal anticancer treatments, and had a qualifying tumor mutation in one of 15 genes involved in the Homologous Recombination Repair (HRR) pathway. Those in Cohort A (N=245) had alterations in BRCA1, BRCA2 or ATM genes while those in Cohort B (N=142) had alterations in any one of 12 other genes known to be involved in DNA repair which included BRIP1, BARD1, CDK12, CHEK1, CHEK2, FANCL, PALB2, PPP2R2A, RAD51B, RAD51C, RAD51D or RAD54L. Patients were randomized 2:1 within each cohort to receive LYNPARZA® 300 mg orally BID or physician’s choice of XTANDI® 160 mg orally QD or ZYTIGA® 1000 mg orally QD along with Prednisone 5 mg orally BID. Patient characteristics were well-balanced between arms in each treatment group, median patient age was 68 years, approximately 25% of patients had de novo metastatic disease, about 65% of patients received prior Taxane therapy and more than 20% had received two lines of chemotherapy. Patients were allowed to cross over to LYNPARZA® upon progression. The Primary endpoint was radiographic Progression-Free Survival (rPFS) in Cohort A, assessed by Blinded Independent Central Review (BICR). Secondary endpoints included Objective Response Rate (ORR), Time to pain progression and Overall Survival (OS) in Cohort A.
It was noted that in Cohort A, the median PFS was 7.4 months with LYNPARZA®, compared to 3.5 months with hormonal treatment (HR=0.34, P<0.0001). This represented a 66% greater delay in disease progression compared to hormonal therapy. In the overall population (Cohort A+B), median PFS was 5.8 months versus 3.5 months respectively (HR=0.49, P<0.0001). The interim Overall Survival analysis in Cohort A showed that median OS was 18.5 months with LYNPARZA® compared to 15 months with hormonal treatment (HR=0.64, P=0.0173). Median OS in the overall population (Cohort A+B) was 17.5 months versus 14.2 months with LYNPARZA® versus hormonal treatment, respectively (HR 0.67, P=0.0063). In Cohort A, the Objective Response Rate (ORR) was 33.3% with LYNPARZA® compared with 2.3% with the hormonal therapies (P<0.0001). The median Time to pain progression was not yet reached with LYNPARZA® compared with 9.9 months for the hormonal agents. This suggested a 56% reduction in the risk of pain progression (HR=0.44; P=0.019). The most common Adverse Events associated with LYNPARZA® were nausea, decreased appetite, anemia and fatigue.
It was concluded that in this landmark, molecularly targeted, Phase III trial, LYNPARZA® improved Progression Free Survival and Objective Response Rates, with a trend for Overall Survival, among patients with heavily pretreated metastatic CRPC, and alterations in the Homologous Recombination Repair genes. PROFOUND: Phase III study of olaparib versus enzalutamide or abiraterone for metastatic castration-resistant prostate cancer (mCRPC) with homologous recombination repair (HRR) gene alterations. Hussain M, Mateo J, Fizazi K, et al. Presented at ESMO Congress 2019; September 27-October 1, 2019; Barcelona, Spain. Abstract LBA12_PR.

