SUMMARY: Prostate cancer is the most common cancer in American men with the exclusion of skin cancer, and 1 in 9 men will be diagnosed with Prostate cancer during their lifetime. It is estimated that in the United States, about 174,650 new cases of Prostate cancer will be diagnosed in 2019 and 31,620 men will die of the disease. The development and progression of Prostate cancer is driven by androgens. Androgen Deprivation Therapy (ADT) or testosterone suppression has therefore been the cornerstone of treatment of advanced Prostate cancer and is the first treatment intervention.
The first-generation NonSteroidal Anti-Androgen (NSAA) agents such as EULEXIN® (Flutamide), CASODEX® (Bicalutamide) and NILANDRON® (Nilutamide) act by binding to the Androgen Receptor (AR) and prevent the activation of the AR and subsequent up-regulation of androgen responsive genes. They may also accelerate the degradation of the AR. These agents have a range of pharmacologic activity from being pure anti-androgens to androgen agonists. CASODEX® is a nonsteroidal oral anti-androgen, that is often prescribed along with GnRH (Gonadotropin-Releasing Hormone) agonists for metastatic disease, or as a single agent second line hormonal therapy for those who had progressed on LHRH agonists. XTANDI® (Enzalutamide) is an orally administered, second-generation, anti-androgen, with no reported agonistic effects. It competitively inhibits androgens and AR binding to androgens as well as AR nuclear translocation and interaction with DNA. It thus inhibits several steps in the AR signaling pathway and was designed to overcome acquired resistance to first-generation nonsteroidal anti-androgens. Previously published studies have shown that XTANDI® improved Overall Survival in Castration-Resistant Prostate Cancer, regardless of whether it was used before or after Docetaxel chemotherapy. The benefits of adding Docetaxel or ZYTIGA® (Abiraterone) to testosterone suppression in men with metastatic, hormone-sensitive Prostate cancer have been established in randomized clinical trials.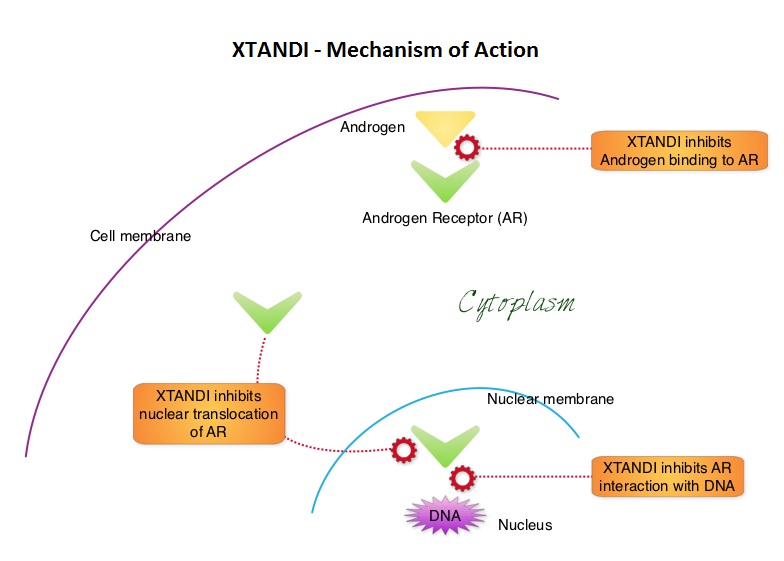
ENZAMET (Enzalutamide in First Line Androgen Deprivation Therapy for Metastatic Prostate Cancer) is an open-label, international, randomized, Phase III trial, conducted to evaluate the benefits of adding XTANDI® to initial standard treatment of Androgen Deprivation Therapy (ADT) with or without early Docetaxel, among patients with metastatic hormone-sensitive Prostate cancer.
A total of 1125 men with metastatic hormone-sensitive Prostate cancer were randomly assigned 1:1 to receive either ADT plus XTANDI® or NonSteroidal Anti-Androgens (NSAA). ADT consisted of parenteral injection of a testosterone-suppressing agent (such as Goserelin, Leuprolide, or Degarelix) with either a 160 mg dose of XTANDI® daily or one of the standard NSAA’s such as CASODEX®, EULEXIN® or NILANDRON®. Of the 1,125 men enrolled in the trial, 503 men received early doses of Docetaxel, and 602 did not. The decision to initiate early treatment with Docetaxel was at the treating physician’s discretion and was administered at 75 mg/m2 IV without prednisone every 3 weeks for a maximum of six cycles. Randomized patients were stratified according to the volume of disease (High Risk- defined as the presence of visceral metastases or at least four bone lesions with at least one lesion located beyond the vertebral bodies and pelvis or low), planned use of early Docetaxel, planned use of bone antiresorptive therapy, and score on ACE-27 (Adult Comorbidity Evaluation 27), with no coexisting conditions rated as 0, mild rated as 1, moderate rated as 2, and severe or multiple conditions rated as 3. The mean age was 68 years, 45% of patients received early Docetaxel as planned treatment and over 50% of the patients had high volume disease. The Primary end point was Overall Survival (OS) and Secondary end points included Progression Free Survival (PFS) as determined by the PSA level, clinical PFS, and adverse events. The median follow up was 34 months.
At the time of the first interim analysis, there was a 33% reduction in the risk of death in the XTANDI® group compared to the standard treatment group ((HR=0.67; P<0.002) and the estimated Overall Survival at 3 years were 80% in the XTANDI® group and 72% in the standard-of-care group. The addition of XTANDI® also improved PSA Progression Free Survival with a 61% reduction in the risk of PSA progression (HR=0.39; P<0.001) and 60% improvement in clinical PFS (HR=0.40; P<0.001). The effects of XTANDI® on clinical PFS were noted in all predefined subgroups, including those with early Docetaxel treatment. Among the patient group who also received early Docetaxel treatment, there was however no significant improvement in Overall Survival. Adding XTANDI® to standard ADT was associated with a higher frequency of toxic effects, especially peripheral neuropathy, associated with the concomitant use of Docetaxel, fatigue and slightly higher risk of seizures compared to standard therapy, and more patients discontinued treatment due to adverse events in the XTANDI® group.
It was concluded that XTANDI® was associated with significantly longer Progression Free Survival and Overall Survival than standard intervention, in men with metastatic, hormone-sensitive Prostate cancer receiving Androgen Deprivation Therapy. Patients who received early Docetaxel treatment, however did not have significant survival benefit. The authors added that ENZAMET is the first metastatic hormone-sensitive Prostate cancer trial to report Overall Survival data of an androgen receptor inhibitor (XTANDI®), and outcomes among a set of patients who also concurrently received Docetaxel. Enzalutamide with Standard First-Line Therapy in Metastatic Prostate Cancer. Davis ID, Martin AJ, Stockler MR, et al. for the ENZAMET Trial Investigators and the Australian and New Zealand Urogenital and Prostate Cancer Trials Group. June 2, 2019. DOI: 10.1056/NEJMoa1903835

