SUMMARY: Tumor genomic profiling enables the identification of specific genomic alterations and thereby can provide personalized treatment options with targeted therapies that are specific for those molecular targets. The FDA in May 2017, granted accelerated approval to KEYTRUDA® (Pembrolizumab), for adult and pediatric patients with unresectable or metastatic, MicroSatellite Instability-High (MSI-H) or MisMatch Repair deficient (dMMR) solid tumors that have progressed following prior treatment and who have no satisfactory alternative treatment options. This is the first FDA approval of a systemic cancer treatment, based on a specific genetic biomarker, independent of tumor origin (first tissue/site-agnostic approval).
A genomic test can be performed on a tumor specimen or on cell-free DNA in plasma (“liquid biopsy”) or an ImmunoHistoChemistry (IHC) test can be performed on tumor tissue for protein expression that demonstrates a genomic variant known to be a drug target, or to predict sensitivity to a chemotherapeutic drug. Next-generation sequencing (NGS) platforms or second-generation sequencing unlike the first-generation sequencing, known as Sanger sequencing, perform massively parallel sequencing, which allows sequencing of millions of fragments of DNA from a single sample. With this high-throughput sequencing, the entire genome can be sequenced in less than 24 hours. Recently reported genomic profiling studies performed in patients with advanced cancer suggest that actionable mutations are found in 20-40% of patients’ tumors.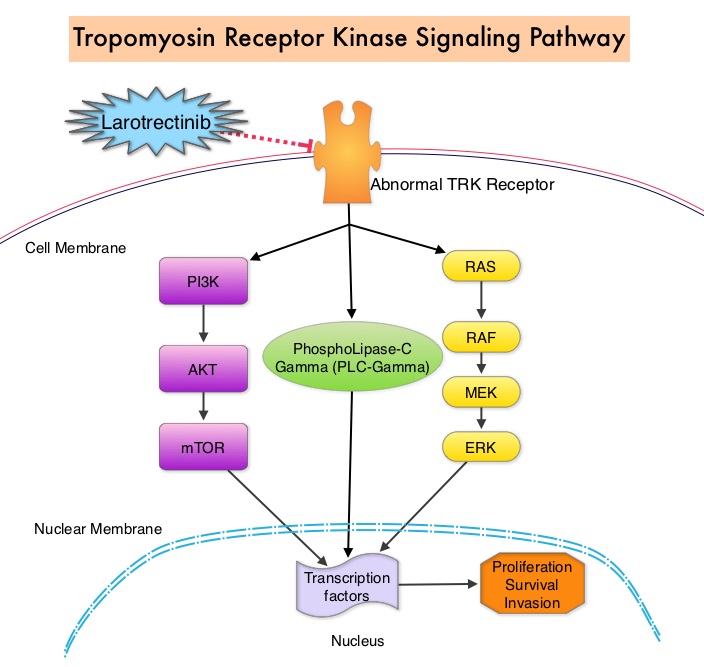
The molecules of interest are Neurotrophic Tropomyosin Receptor Kinase (NTRK) gene rearrangements. The three TRK family of Tropomyosin Receptor Kinase (TRK) transmembrane proteins TRKA, TRKB, and TRKC are encoded by Neurotrophic Tropomyosin Receptor Kinase genes NTRK1, NTRK2, and NTRK3, respectively. These receptor tyrosine kinases are expressed in human neuronal tissue and are involved in a variety of signaling events such as cell differentiation, cell survival and apoptosis of peripheral and central neurons. They therefore play an essential role in the physiology of development and function of the nervous system. Chromosomal fusion involving NTRK genes represent the main molecular alterations with known oncogenic and transforming potential and have been identified in a variety of cancers both in children and adults. Gene fusions involving NTRK genes lead to transcription of chimeric TRK proteins which can confer oncogenic potential by increasing cell proliferation and survival. These genetic abnormalities have generated a lot of interest and have emerged as targets for cancer therapy. NGS has allowed the discovery of these gene fusions. Early clinical evidence suggests that these gene fusions lead to oncogene addiction regardless of tissue of origin. (Oncogene addiction is the dependency of some cancers on one or a few genes for the maintenance of the malignant phenotype).
Larotrectinib is a potent and highly selective small molecule inhibitor of all three TRK proteins. The authors in this development program included patients of any age and with any tumor type who had chromosomal fusion involving NTRK genes (Age and Tumor agnostic therapy). This program enrolled 55 patients, ranging in age from 4 months to 76 years, with consecutively and prospectively identified TRK fusion-positive cancers, detected by molecular profiling. They were assigned to three clinical studies – a phase I study involving adults, a phase I-II study involving children and a phase II study involving adolescents and adults.
This population of patients encompassed 17 unique cancer diagnoses and enrolled patients had locally advanced or metastatic solid tumors, and had received prior standard therapy (if available). The Primary end point for the combined analysis was the Overall Response Rate according to Independent review. Secondary end points included Duration of Response, Progression Free Survival, and safety. The researchers in this publication reported the safety and efficacy analysis of the first 55 consecutively enrolled patients, identified with TRK fusion-positive cancers, treated across these studies.
The Overall Response Rate was 75%. A total of 13% of the patients had a Complete Response, 62% had a Partial Response and 13% had stable disease. The median Duration of Response and Progression Free Survival had not been reached. At a median follow-up of 9.4 months, 86% of the patients with a response continued treatment or had undergone curative surgery. At 1 year, 71% of the responses were ongoing and 55% of the patients remained progression-free. The most common adverse events of any grade were fatigue, vomiting and abnormal liver function studies. None of the patients on Larotrectinib discontinued therapy due to a drug-related adverse event.
It was concluded that TRK fusions defined a unique molecular subgroup of advanced solid tumors in children and adults and Larotrectinib had marked and durable antitumor activity in patients with TRK fusion-positive cancer, regardless of the age of the patient or tumor type. Efficacy of Larotrectinib in TRK Fusion–Positive Cancers in Adults and Children. Drilon A, Laetsch TW, Kummar S, et al. N Engl J Med 2018; 378:731-739

