SUMMARY: Breast cancer is the most common cancer among women in the US and about 1 in 8 women (12%) will develop invasive breast cancer during their lifetime. Approximately 290,560 new cases of breast cancer will be diagnosed in 2022 and about 43,780 individuals will die of the disease, largely due to metastatic recurrence. Approximately 70% of breast tumors express Estrogen Receptors and/or Progesterone Receptors and these patients are often treated with anti-estrogen therapy as first line treatment. However, resistance to hormonal therapy occurs in a majority of the patients.
Cyclin Dependent Kinases (CDK) play a very important role to facilitate orderly and controlled progression of the cell cycle. Genetic alterations in these kinases and their regulatory proteins have been implicated in various malignancies. Cyclin Dependent Kinases 4 and 6 (CDK4 and CDK6) phosphorylate RetinoBlastoma protein (RB), and initiate transition from the G1 phase to the S phase of the cell cycle. RetinoBlastoma protein has antiproliferative and tumor-suppressor activity and phosphorylation of RB protein nullifies its beneficial activities. CDK4 and CDK6 are activated in hormone receptor positive breast cancer, promoting breast cancer cell proliferation. Further, there is evidence to suggest that endocrine resistant breast cancer cell lines depend on CDK4 for cell proliferation and associated with increased expression of CDK4. The understanding of the role of Cyclin Dependent Kinases in the cell cycle, has paved the way for the development of CDK inhibitors.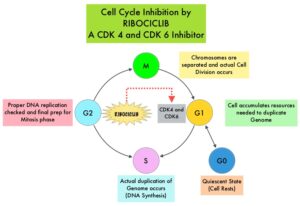
KISQALI® (Ribociclib) is an orally bioavailable, selective, small-molecule inhibitor of CDK4/6, preferentially inhibiting CDK4, that blocks the phosphorylation of RetinoBlastoma protein, thereby preventing cell-cycle progression and inducing G1 phase arrest. In a phase 1b study involving postmenopausal women with ER positive, HER2-negative advanced breast cancer, KISQALI® in combination with FEMARA® (Letrozole) demonstrated an Overall Response Rate (ORR) of 46% and a Clinical Benefit Rate of 79%, in treatment-naïve patients with advanced breast cancer. This led to the design of MONALEESA-2 trial.
MONALEESA-2 trial is a randomized, double-blind, placebo-controlled, Phase III study in which 668 patients were randomly assigned in a 1:1 ratio to receive either KISQALI® plus FEMARA® or placebo plus FEMARA®. Eligible patients included post-menopausal women with HR-positive, HER2-negative advanced or metastatic breast cancer who had received no prior therapy for advanced disease. Treatment consisted of oral KISQALI® 600 mg daily on a 3-weeks on and 1-week off schedule, in 28-day treatment cycles plus FEMARA® 2.5 mg orally daily on a continuous schedule or placebo plus FEMARA®. Patients were stratified according to the presence or absence of liver or lung metastases and treatment was continued until disease progression or unacceptable toxicity. No treatment crossover was allowed. The median age was 62 years, close to 60% of the patients had visceral metastases, and patients were stratified according to the presence or absence of liver or lung metastases. The Primary end point was Progression Free Survival (PFS) and Secondary end points included Overall Survival (OS), Overall Response Rate (ORR), Clinical Benefit Rate (Overall Response plus Stable disease lasting 24 weeks or more), Safety, and Quality of Life assessments.
In the primary and updated analyses of the MONALEESA-2 trial, PFS was significantly longer with KISQALI® plus FEMARA® than with placebo plus FEMARA® (25.3 months versus 16.0 months; HR for disease progression or death=0.57; P<0.001). The Overall Survival data were immature at the time of the primary and updated analyses. The authors have now reported the findings from the protocol-specified final analysis of Overall Survival, which is a key Secondary end point.
After a median follow up of 6.6 years, a significant Overall Survival benefit was observed with KISQALI® plus FEMARA®, compared to placebo plus FEMARA®. The median Overall Survival was 63.9 months with KISQALI® plus FEMARA® and 51.4 months with placebo plus FEMARA® (HR=0.76; two-sided P=0.008). This Overall Survival benefit was consistent across all prespecified subgroups. The median time to first subsequent chemotherapy was 50.6 months in the KISQALI® group and 38.9 months in the placebo group (HR for receipt of first chemotherapy=0.74). No new safety signals were observed.
It was concluded from the analysis of the MONALEESA-2 trial that first line therapy with KISQALI® plus FEMARA® showed a significant Overall Survival benefit as compared with placebo plus FEMARA®, in patients with HR-positive, HER2-negative advanced breast cancer, with a 24% relative reduction in the risk of death. The authors added that MONALEESA trials of KISQALI® have shown a consistent Overall Survival benefit regardless of accompanying endocrine therapy, line of therapy, or menopausal status.
Overall Survival with Ribociclib plus Letrozole in Advanced Breast Cancer. Hortobagyi GN, Stemmer SM, Burris HA, et al. N Engl J Med 2022; 386:942-950

