SUMMARY: Prostate cancer is the most common cancer in American men, excluding skin cancer and 1 in 7 men will be diagnosed with prostate cancer during their lifetime. It is estimated that in the United States, over 230,000 new cases of prostate cancer will be diagnosed in 2014 and close to 30,000 men will die of the disease.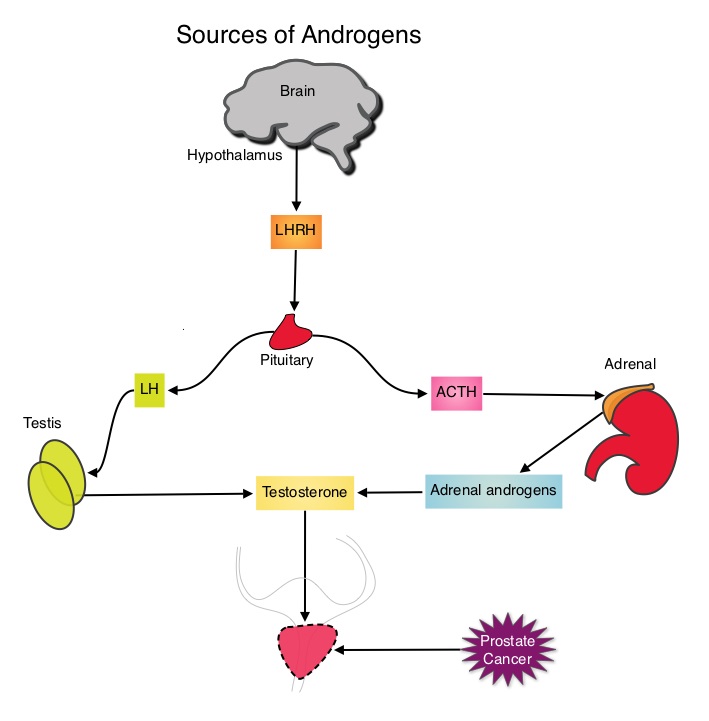 The development and progression of prostate cancer is driven by androgens. Androgen Deprivation Therapy (ADT) has therefore been the cornerstone of treatment of advanced prostate cancer and is the first treatment intervention for hormone sensitive prostate cancer. Chemotherapy is usually considered for patients who progress on hormone therapy and TAXOTERE® (Docetaxel) has been shown to improve Overall Survival (OS) of metastatic prostate cancer patients, who had progressed on androgen deprivation therapy. It is not clear however, whether ADT is more effective with or without TAXOTERE®, when treating patients with metastatic prostate cancer. To address this further, a randomized phase III trial (E3805) was conducted to assess the benefit of upfront treatment with a combination of chemotherapy and hormonal therapy, in patients with metastatic hormone sensitive prostate cancer. Seven hundred and ninety (N=790) patients with newly diagnosed metastatic prostate cancer were randomly assigned to receive either Androgen Deprivation Therapy alone (N=393) or ADT plus TAXOTERE® (N=397). Androgen Deprivation Therapy consisted of either Luteinizing Hormone Releasing Hormone (LHRH) agonist therapy, LHRH antagonist therapy, or surgical castration. Chemotherapy consisted of TAXOTERE®, started within 4 months of starting ADT, dosed at 75 mg/m2 given every 3 weeks for a maximum of six cycles. The median age of patients was 63 years and approximately two-thirds of patients had high-volume disease, with either extensive liver or bone metastases. The primary endpoint of this study was Overall Survival. At a median follow up of 29 months, the median Overall Survival was 42.3 months in the ADT group and 52.7 months in the ADT plus TAXOTERE® group (HR=0.63; P<0.0006). This benefit was even more significant in patients with high volume disease (32.2 vs 49.2 months for ADT and ADT plus TAXOTERE® respectively, HR=0.62; P<0.0012). At 12 months, the proportion of patients with PSA levels less than 0.2 ng/mL was 9.4% in the ADT alone group vs 19.7% in the ADT plus TAXOTERE® group (P < 0.0001). The median time to clinical progression was 19.8 months in the ADT alone group vs 32.7 months in the ADT plus TAXOTERE® group (P < 0.0001). The authors concluded that this is the first study to demonstrate survival benefit in patients with newly diagnosed metastatic prostate cancer. This survival benefit with Androgen Deprivation Therapy and TAXOTERE® is even more so, in patients with high volume disease and should be considered standard treatment for those patients who are fit to receive TAXOTERE® based therapy. Sweeney C, Chen Y, Carducci MA, et al. 2014 ASCO Annual Meeting; LBA2
The development and progression of prostate cancer is driven by androgens. Androgen Deprivation Therapy (ADT) has therefore been the cornerstone of treatment of advanced prostate cancer and is the first treatment intervention for hormone sensitive prostate cancer. Chemotherapy is usually considered for patients who progress on hormone therapy and TAXOTERE® (Docetaxel) has been shown to improve Overall Survival (OS) of metastatic prostate cancer patients, who had progressed on androgen deprivation therapy. It is not clear however, whether ADT is more effective with or without TAXOTERE®, when treating patients with metastatic prostate cancer. To address this further, a randomized phase III trial (E3805) was conducted to assess the benefit of upfront treatment with a combination of chemotherapy and hormonal therapy, in patients with metastatic hormone sensitive prostate cancer. Seven hundred and ninety (N=790) patients with newly diagnosed metastatic prostate cancer were randomly assigned to receive either Androgen Deprivation Therapy alone (N=393) or ADT plus TAXOTERE® (N=397). Androgen Deprivation Therapy consisted of either Luteinizing Hormone Releasing Hormone (LHRH) agonist therapy, LHRH antagonist therapy, or surgical castration. Chemotherapy consisted of TAXOTERE®, started within 4 months of starting ADT, dosed at 75 mg/m2 given every 3 weeks for a maximum of six cycles. The median age of patients was 63 years and approximately two-thirds of patients had high-volume disease, with either extensive liver or bone metastases. The primary endpoint of this study was Overall Survival. At a median follow up of 29 months, the median Overall Survival was 42.3 months in the ADT group and 52.7 months in the ADT plus TAXOTERE® group (HR=0.63; P<0.0006). This benefit was even more significant in patients with high volume disease (32.2 vs 49.2 months for ADT and ADT plus TAXOTERE® respectively, HR=0.62; P<0.0012). At 12 months, the proportion of patients with PSA levels less than 0.2 ng/mL was 9.4% in the ADT alone group vs 19.7% in the ADT plus TAXOTERE® group (P < 0.0001). The median time to clinical progression was 19.8 months in the ADT alone group vs 32.7 months in the ADT plus TAXOTERE® group (P < 0.0001). The authors concluded that this is the first study to demonstrate survival benefit in patients with newly diagnosed metastatic prostate cancer. This survival benefit with Androgen Deprivation Therapy and TAXOTERE® is even more so, in patients with high volume disease and should be considered standard treatment for those patients who are fit to receive TAXOTERE® based therapy. Sweeney C, Chen Y, Carducci MA, et al. 2014 ASCO Annual Meeting; LBA2

