SUMMARY: The American Cancer Society estimates that in 2019, 21,450 new cases of Acute Myeloid Leukemia (AML) will be diagnosed in the United States and 10,920 patients will die of the disease. AML can be considered as a group of heterogeneous diseases with different clinical behavior and outcomes. A significant percentage of patients with newly diagnosed AML are not candidates for intensive chemotherapy. Even with the best available therapies, the 5 year Overall Survival in patients 65 years of age or older is less than 5%. Cytogenetic analysis has been part of routine evaluation when caring for patients with AML. By predicting resistance to therapy, tumor cytogenetics will stratify patients, based on risk and help manage them accordingly. Even though cytotoxic chemotherapy may lead to long term remission and cure in a minority of patients with favorable cytogenetics, patients with high risk features such as unfavorable cytogenetics, molecular abnormalities, prior myelodysplasia and advanced age, have poor outcomes with conventional chemotherapy alone. More importantly, with the understanding of molecular pathology of AML, personalized and targeted therapies are becoming an important part of the AML treatment armamentarium.
Isocitrate DeHydrogenase (IDH) is a metabolic enzyme that helps generate energy from glucose and other metabolites, by catalyzing the conversion of Isocitrate to Alpha-Ketoglutarate. Alpha-ketoglutarate is required to properly regulate DNA and histone methylation, which in turn is important for gene expression and cellular differentiation. IDH mutations lead to aberrant DNA methylation and altered gene expression thereby preventing cellular differentiation, with resulting immature undifferentiated cells. IDH mutations can thus promote leukemogenesis in Acute Myeloid Leukemia and tumorigenesis in solid tumors and can result in inferior outcomes. There are three isoforms of IDH. IDH1 is mainly found in the cytoplasm, as well as in peroxisomes, whereas IDH2 and IDH3 are found in the mitochondria, and are a part of the Krebs cycle. Approximately 20% of patients with AML, 70% of patients with Low-grade Glioma and secondary Glioblastoma, 50% of patients with Chondrosarcoma, 20% of patients with Intrahepatic cholangiocarcinoma, 30% of patients with Angioimmunoblastic T-cell lymphoma and 8% of patients with Myelodysplastic syndromes/Myeloproliferative neoplasms, are associated with IDH mutations.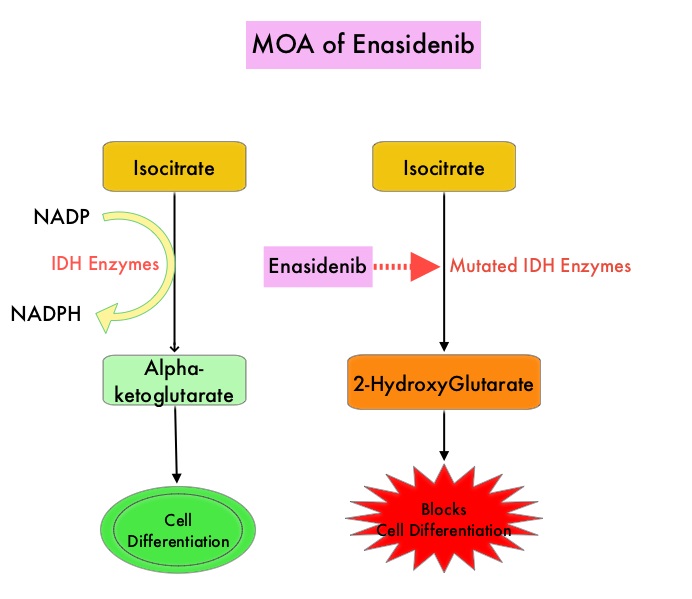
IDHIFA® (Enasidenib) is a selective, oral, small molecule inhibitor of mutated IDH2 protein that promotes myeloid cell differentiation. IDHIFA® indirectly reduces DNA methylation by suppressing the oncometabolite, 2-HydroxyGlutarate, thereby restoring function to Alpha-Ketoglutarate-dependent TET family enzymes. IDHIFA® was approved in the US in 2017 for the treatment of adult patients with relapsed or refractory Acute Myeloid Leukemia (AML), with an IDH2 mutation. Further, treatment with single agent IDHIFA® resulted in an Overall Response Rate (ORR) of 31% and a Completer Response (CR) rate of 18% in patients with newly diagnosed AML. VIDAZA® (Azacitidine) is a hypomethylating agent that promotes DNA hypomethylation by inhibiting DNA methyltransferases. VIDAZA® has been shown to significantly improve Overall Survival (OS) when compared to conventional care regimens in elderly unfit patients with newly diagnosed AML, who are not candidates for intensive chemotherapy. In vitro studies demonstrated that a combination of IDHIFA® and VIDAZA® enhance cell differentiation and apoptosis.
Based on this preclinical data and early clinical trials, an open label, Phase I/II study was conducted comparing a combination of IDHIFA® and VIDAZA® with single agent VIDAZA® in patients with newly diagnosed IDH2 mutated AML, who are not candidates for intensive chemotherapy. The authors reported the first interim outcomes from the randomized, Phase II portion of this ongoing study. The Phase II portion of the trial enrolled 101 patients with newly diagnosed IDH2-mutant AML who were ineligible to receive intensive chemotherapy. Patients had an ECOG PS score of 2 or less and were randomized in a 2:1 ratio to receive IDHIFA® plus VIDAZA® or VIDAZA® alone in repeated 28-day cycles. All patients received VIDAZA® 75 mg/m2/day SC for the first 7 days of each treatment cycle, whereas patients randomized to IDHIFA® plus VIDAZA® also received IDHIFA® 100 mg orally QD continuously. The median patient age was 75 years, and 78% in the combination group and 90% in the VIDAZA® only group had intermediate-risk cytogenetics respectively, and 18% and 10% had poor-risk cytogenetics. The median number of treatment cycles was 8. The Primary endpoint was Overall Response Rate (ORR), which included Complete Remission (CR), CR with incomplete blood or platelet count recovery (CRi/CRp), Partial Remission (PR), and Morphologic Leukemia-Free State (MLFS), per modified IWG 2003 AML response criteria. Mutant IDH2 Variant Allele Frequencies (VAF) in bone marrow mononuclear cells was assessed by digital PCR.
It was noted that the ORR were significantly higher with combination treatment vs VIDAZA® alone (71% versus 42% respectively, P=0.0064) and the CR rates were 53% versus 12% (P=0.0001). The time to first response was about 2 months in each treatment group and the median Duration of Response was 24.1 months with the combination treatment and 12.1 months with VIDAZA® alone. Responses were observed in patients with RAS pathway co-mutations, which have been usually associated with resistance to IDHIFA® monotherapy. The maximal mutant IDH2 VAF suppression from baseline was significantly greater with the combination treatment versus single agent VIDAZA® (median –69.3% versus –14.1% respectively, P=0.0004). Treatment related Grade 3-4 Adverse Events occurring in 10% or more of patients in the combination group were neutropenia, thrombocytopenia, anemia, febrile neutropenia and IDH differentiation syndrome.
It was concluded that a combination of IDHIFA® plus VIDAZA® was associated with significantly improved Complete Remission and Overall Response Rates, with significant mutant IDH2 Variant Allele Frequencies reductions, compared with VIDAZA® alone, in patients with newly diagnosed IDH2-mutant AML. Further the combination treatment was generally well tolerated, with a safety profile similar to that reported for monotherapy with either of these two agents. Enasidenib Plus Azacitidine Significantly Improves Complete Remission and Overall Response Compared with Azacitidine Alone in Patients with Newly Diagnosed Acute Myeloid Leukemia (AML) with Isocitrate dehydrogenase 2 (IDH2) Mutations: Interim Phase II Results from an Ongoing, Randomized Study. DiNardo CD, Schuh AC, Stein EM, et al. Presented at 2019 ASH Annual Meeting; December 7-10, 2019; Orlando, FL. Abstract 643.

