SUMMARY: The Centers for Disease Control and Prevention (CDC) estimates that there are 800,000 -1.4 million individuals with Chronic Hepatitis B (HBV) infection in the United States. Reactivation of HBV is a major concern in cancer patients who may be on chemotherapy or other immunosuppressive therapies, with the incidence of HBV reactivation ranging from 40%-60% in those who are positive for Hepatitis B surface antigen (HBsAg). HBV reactivation is preventable with prophylactic antiviral therapy, failing which it can result in delays in cancer treatment, as well as potentially fatal outcomes. Based on recently published data, showing the high risk for HBV reactivation among patients with hematological malignancies receiving B-cell-depleting agents such as RITUXAN® (Rituximab) or ARZERRA® (Ofatumumab), the FDA has urged health care providers to screen all patients for HBV infection, prior to starting therapy with these agents. HBV reactivation has been observed following chemotherapy for solid tumors, but the risk for reactivation in these settings has been unclear with insufficient evidence. The American Society of Clinical Oncology in 2010 rendered a Provisional Clinical Opinion (PCO), suggesting that there was insufficient evidence to determine the net benefits and harms of routine screening for HBV infection, in patients receiving chemotherapy and the recommendation was that screening be considered in those at increased risk for HBV infection or who receive highly immunosuppressive regimens.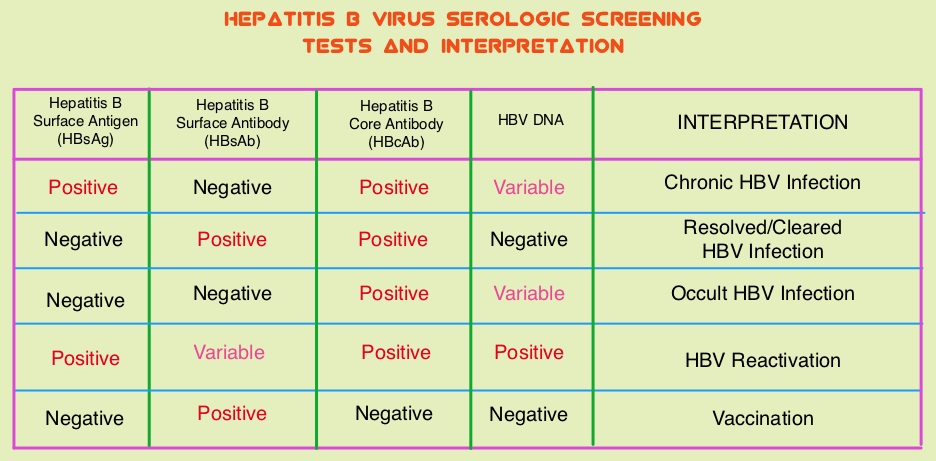
This present study was conducted to determine the risk for HBV reactivation with and without antiviral prophylaxis and the benefit of prophylaxis in adults with solid tumors and chronic or resolved HBV infection. This meta-analysis included 26 original reports and the studies were independently reviewed by two investigators for study inclusion. HBV patients included in this study were receiving chemotherapy for any solid tumor with or without concomitant HBV prophylactic therapy. Study patients could receive long-term antiviral treatment or prophylaxis before chemotherapy initiation and the comparison was with those receiving chemotherapy without antiviral prophylaxis. The primary outcome was HBV reactivation as defined by a greater than 10-fold increase in HBV DNA levels from baseline or an absolute increase greater than 105 copies/mL in those with chronic HBV infection or the re-emergence of HBsAg when previously negative, in those with resolved HBV infection. Secondary outcomes included HBV-related hepatitis, interruption or delay in chemotherapy, acute liver failure with coagulopathy and hepatic encephalopathy and death.
It was noted that in patients with chronic HBV infection receiving chemotherapy, the risk for HBV reactivation without antiviral prophylaxis ranged from 4% to 68% (median, 25%). The risk for HBV reactivation, HBV-related hepatitis, and chemotherapy interruption was reduced by more than 80% with antiviral prophylaxis. Interestingly, in patients with resolved HBV infection receiving chemotherapy, there was still a risk of HBV reactivation, with this risk ranging from 0.3% to 9%. The authors in this meta-analysis addressed a very important question and concluded that the risk for HBV reactivation in patients with chronic HBV, on chemotherapy for solid tumors, is similar to the risk with other types of immunosuppressive therapy. Cancer patients should therefore be screened for HBV before chemotherapy is initiated for solid tumors and started on antiviral prophylaxis. Paul S, Saxena A, Terrin N, et al. Hepatitis B Virus Reactivation and Prophylaxis During Solid Tumor Chemotherapy: A Systematic Review and Meta-analysis. Ann Intern Med. 2016; 164:30-40.

