SUMMARY: The FDA on April 11, 2019, approved KEYTRUDA® (Pembrolizumab) for the first-line treatment of patients with Stage III Non-Small Cell Lung Cancer (NSCLC) who are not candidates for surgical resection or definitive chemoradiation, as well as those with metastatic NSCLC. Patients’ tumors must have no EGFR or ALK genomic aberrations and express PD-L1 (Tumor Proportion Score-TPS of 1% or more), as determined by an FDA-approved test. Lung cancer is the second most common cancer in both men and women and accounts for about 14% of all new cancers and 27% of all cancer deaths. The American Cancer Society estimates that for 2019 about 228,150 new cases of lung cancer will be diagnosed and 142,670 patients will die of the disease. Lung cancer is the leading cause of cancer-related mortality in the United States. Non-Small Cell Lung Cancer (NSCLC) accounts for approximately 85% of all lung cancers. Of the three main subtypes of NSCLC, 30% are Squamous Cell Carcinomas (SCC), 40% are Adenocarcinomas and 10% are Large Cell Carcinomas.
KEYTRUDA® (Pembrolizumab) is a fully humanized, Immunoglobulin G4, anti-PD-1, monoclonal antibody, that binds to the PD-1 receptor and blocks its interaction with ligands PD-L1 and PD-L2. It thereby reverses the PD-1 pathway-mediated inhibition of the immune response and unleashes the tumor-specific effector T cells. High level of Programmed Death-Ligand 1 (PD-L1) expression is defined as membranous PD-L1 expression on at least 50% of the tumor cells, regardless of the staining intensity. It is estimated that based on observations from previous studies, approximately 25% of the patients with advanced NSCLC have a high level of PD-L1 expression, and high level of PD-L1 expression has been associated with significantly increased response rates to KEYTRUDA®. The FDA approved KEYTRUDA® for the first-line treatment of advanced NSCLC with high PD-L1 expression (Tumor Proportion Score of 50% or more), based on KEYNOTE-024 trial, as well as in combination with Pemetrexed and Carboplatin, as first-line treatment of patients with metastatic non-squamous NSCLC, based on KEYNOTE-021 study. It is also indicated for previously treated advanced NSCLC with a much lower level of PD-L1 expression such as PD-L1 Tumor Proportion Score of 1% or higher, based on KEYNOTE-010 trial.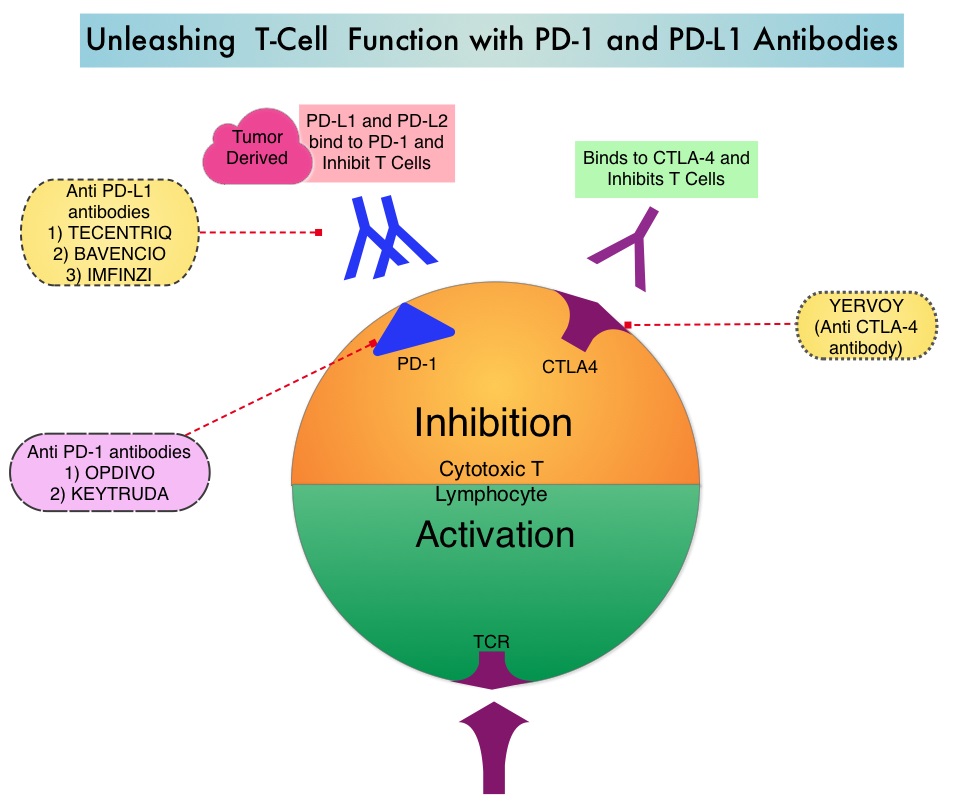
KEYNOTE-042 is a large, international, multicenter, randomized phase III trial in which 1274 patients with untreated locally advanced or metastatic NSCLC were randomly assigned to KEYTRUDA® or chemotherapy with Paclitaxel plus Carboplatin or Pemetrexed plus Carboplatin. In this study, both squamous and non-squamous cancers with PD-L1 Tumor Proportion Score (TPS) of 1% or more were included, but tumors with sensitizing Epidermal Growth Factor Receptor (EGFR) or Anaplastic Lymphoma Kinase (ALK) mutations cancers with genetic changes, that could be treated with targeted therapies such as EGFR and ALK inhibitors, were excluded. Eligible patients were randomly assigned in a 1:1 to receive either KEYTRUDA® 200 mg IV every 3 weeks for up to 35 cycles or investigator’s choice of up to 6 cycles of chemotherapy with Paclitaxel plus Carboplatin or Pemetrexed plus Carboplatin, with optional Pemetrexed maintenance for non-squamous NSCLC. Patients were divided into 3 treatment groups based on their PD-L1 Tumor Proportion Score (TPS): TPS 50% or more (N=599), TPS 20% or more (N=818), and TPS 1% or more (N=637). Each PD-L1 expression group had equal numbers of patients receiving KEYTRUDA® and chemotherapy. The Primary end points were Overall Survival (OS) in patients with TPS 50% or more, 20% or more, and 1% or more.
At a median follow up of 12.8 months, 13.7% of patients were still receiving KEYTRUDA® compared with 4.9% on Pemetrexed maintenance therapy. It was noted that KEYTRUDA® was significantly superior to chemotherapy in all PD-L1 expression subsets. In patients with a PD-L1 TPS 50% or more, the median OS with KEYTRUDA® was 20 months versus 12.2 months for chemotherapy (HR=0.69, P=0.0003), for patients with PD-L1 TPS 20% or more, the median OS was 17.7 months versus 13 months respectively (HR=0.77, P=0.002), and for those with PD-L1 TPS 1% or more, the median OS was 16.7 months versus 12.1 months respectively (HR=0.81, P = 0.0018). The Response Rates (RR) were also higher among patients who received KEYTRUDA®, with RR of 39.5% for KEYTRUDA® versus 32% for chemotherapy in patients with a TPS 50% or more, 33.4% and 28.9% respectively in patients with TPS 20% or more and 27.3% and 26.5%, respectively, among patients with TPS of 1% or more. The duration of response was also superior with KEYTRUDA® in all three PD-L1 subgroups compared to chemotherapy (20.2 months versus 8-11 months). Patients receiving KEYTRUDA® experienced fewer severe Adverse Events, compared with chemotherapy (17.8% versus 41%).
The authors concluded that this is the largest clinical trial of KEYTRUDA® as a stand-alone therapy, and is the first study with a Primary end point of OS to demonstrate superiority of KEYTRUDA® over platinum-based chemotherapy, in patients with previously untreated locally advanced/metastatic NSCLC, without sensitizing EGFR or ALK alterations and a PD-L1 TPS of 1% or more. These data confirmed the benefit of KEYTRUDA® monotherapy as a standard first-line treatment, for PD-L1-expressing locally advanced Stage III as well as metastatic NSCLC. KEYTRUDA® monotherapy is now a new treatment option for more patients with NSCLC, including those for whom combination therapy may not be appropriate. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Mok TS, Wu Y-L, Kudaba I, et al. The Lancet. Published: April 04, 2019. DOI: https://doi.org/10.1016/S0140-6736(18)32409-7

