SUMMARY: The FDA on May 6, 2020, granted accelerated approval to TABRECTA® (Capmatinib) for adult patients with metastatic Non-Small Cell Lung Cancer (NSCLC), whose tumors have a mutation that leads to Mesenchymal-Epithelial Transition (MET) exon 14 skipping, as detected by an FDA-approved test. The FDA also approved the FoundationOne CDx assay (Foundation Medicine, Inc.) as a companion diagnostic for TABRECTA®.
MET is a widely expressed Receptor Tyrosine Kinase and plays a pivotal role in cell growth, proliferation and survival. The MET gene encodes for a protein known as the Hepatocyte Growth Factor (HGF) Receptor. Upon binding by Hepatocyte Growth Factor (HGF), the HGF Receptor is activated, with resulting activation of the downstream RAS/RAF/MEK/ERK and PI3K/AKT/mTOR signaling pathways, thereby serving different important biological functions. Alterations in the MET gene leading to abnormal MET signaling, has been identified in different types of cancers including thyroid, lung, breast, liver, colon, kidney, ovary and gastric carcinoma.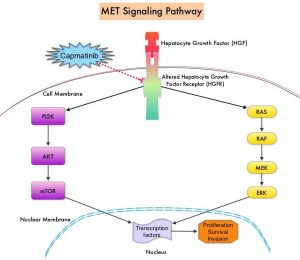
Two key MET alterations include MET exon 14 skipping mutations and MET amplification. MET exon 14 skipping mutations occur in approximately 5% of NSCLC patients with enrichment in sarcomatoid lung cancers (22%). MET exon 14 skipping mutation is a recognized oncogenic driver and is a molecular genetic abnormality indicating the presence of a splice site mutation that results in a loss of transcription of exon 14 of the MET gene. Most exon 14 mutations occur in never-smokers and is seen in both squamous and adenocarcinoma histology. Patients whose cancers have MET exon 14 skipping generally have very high response rates to MET inhibitors and molecular testing for MET exon 14 skipping should therefore be performed on all lung cancers, because this is a targetable alteration. MET amplification has been more commonly seen in smokers, and responses in patients with MET-amplified tumors might be more variable and dependent on level of amplification, with higher responses noted in tumors with more than 5-6 fold amplification. Tumors with MET exon 14 skipping mutations usually do not harbor activating mutations in EGFR, KRAS, or BRAF or concurrent ALK, ROS1 or RET translocations. However, it appears that cMET exon 14 skipping is not mutually exclusive with cMET amplification.
TABRECTA® (Capmatinib) is a highly potent and selective, reversible inhibitor of MET tyrosine kinase. The present FDA approval was based on the primary findings from the Phase II GEOMETRY mono-1 trial, which is a non-randomized, open-label, multi-cohort, Phase II study, conducted to evaluate the efficacy and safety of single-agent TABRECTA® in adult patients with EGFR wild-type, ALK-negative, metastatic NSCLC, whose tumors have a mutation that leads to MET exon 14 skipping (METex14), as detected by an RNA-based RT-PCR. This study enrolled 97 patients with metastatic NSCLC and confirmed MET exon 14 skipping mutations, 69 of whom were previously treated and, 28 of whom, were treatment naive. The patients received TABRECTA® at 400 mg orally twice daily until disease progression or unacceptable toxicity. The median patient age was 71 years and all NSCLC histologies including sarcomatoid/carcinosarcoma were included. Majority of the patients (75%) were white and 24% were Asian. Previous treatments included immunotherapy (28%) and chemotherapy (94%), and 23% of patients received 2 prior lines of therapy. The main efficacy outcome was Overall Response Rate (ORR) and additional efficacy outcomes included Duration of Response, Time to Response, Disease Control Rate, Progression Free Survival (PFS) and Safety. Thirteen patients (N=13) in this study had brain metastases at baseline.
Among the treatment-naïve patients group, the ORR was 68% with a median Duration of Response of 12.6 months and the percentage of patients with responses for 12 months or longer was 47%. The Disease Control Rate (Complete Response plus Partial Response plus Stable Disease) was 96.4%.
Among the previously treated patients, the ORR was 41%, with a median Duration of Response of 9.7 months and the percentage of patients with responses for 12 months or longer was 32%. The Disease Control Rate was 78.3%. Among those with brain metastases at baseline, 54% had an intracranial response with TABRECTA® with 31% showing complete resolution, 23% showing partial resolution, and the intracranial Disease Control Rate was 92%. The most common adverse events (occurring in at least 20% of patients) were peripheral edema, nausea, fatigue, vomiting, dyspnea, and decreased appetite. TABRECTA® can also cause Interstitial Lung Disease, hepatotoxicity and photosensitivity.
It was concluded that TABRECTA® is a new treatment option for patients with MET exon 14 skipping- mutated advanced NSCLC, regardless of the line of therapy, with deep and durable responses, manageable toxicity profile, and is the first and only FDA approved treatment for this patient group.
Capmatinib (INC280) in METex14-mutated advanced non-small cell lung cancer (NSCLC): Efficacy data from the phase II GEOMETRY mono-1 study. Wolf J, Seto T, Han J, et al. J Clin Oncol. 2019;37(suppl; abstr 9004).

