SUMMARY: The FDA on October 2, 2020, approved the combination of OPDIVO® (Nivolumab) plus YERVOY® (Ipilimumab), as first-line treatment for adult patients with unresectable Malignant Pleural Mesothelioma. It is estimated that about 3,000 new Malignant Mesothelioma cases are diagnosed each year. Mesothelioma is more common in Whites and Hispanics/Latinos than in African Americans or Asian Americans, and is also much more common in older people. Mesothelioma is more common in men than in women and the average age at the time of diagnosis for pleural mesothelioma is 72 years. The main risk factor for pleural mesothelioma is exposure to high levels of asbestos, usually in the workplace. Malignant Pleural Mesothelioma is relatively rare and has limited treatment options. The five-year survival rate for patients diagnosed with advanced disease is approximately 10 percent. There is currently only one FDA-approved first-line treatment, and there remains an unmet need for this patient group.
Immune checkpoints are cell surface inhibitory proteins/receptors that are expressed on activated T cells. They harness the immune system and prevent uncontrolled immune reactions by switching off the immune system T cells. Immune checkpoint proteins/receptors include CTLA-4 (Cytotoxic T-Lymphocyte Antigen 4, also known as CD152) and PD-1(Programmed cell Death 1). Checkpoint inhibitors unleash the T cells resulting in T cell proliferation, activation, and a therapeutic response.OPDIVO® is a fully human, immunoglobulin G4 monoclonal antibody that binds to the PD-1 receptor and blocks its interaction with PD-L1 and PD-L2, thereby undoing PD-1 pathway-mediated inhibition of the immune response and unleashing the T cells. YERVOY® is a fully human immunoglobulin G1 monoclonal antibody that blocks Immune checkpoint protein/receptor CTLA-4.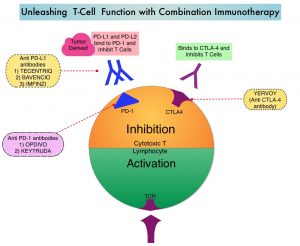
The present FDA approval was based on a prespecified interim analysis of CheckMate 743 trial. This study is an open label, multi-center, randomized Phase III trial, evaluating OPDIVO® plus YERVOY® compared to chemotherapy (Pemetrexed and Cisplatin or Carboplatin), in patients with previously untreated Malignant Pleural Mesothelioma. This study enrolled 605 patients with unresectable pleural mesothelioma and patients were randomized 1:1 to receive either OPDIVO® 3 mg/kg IV once every 2 weeks, in combination with YERVOY® 1 mg/kg IV once every 6 weeks, for up to 2 years (N=303), or six cycles of combination chemotherapy with Cisplatin or Carboplatin plus Pemetrexed every 3 weeks (N=302). The Primary endpoint of the trial was Overall Survival (OS). Secondary endpoints included Objective Response Rate (ORR), Disease Control Rate (DCR), Progression Free Survival (PFS) and efficacy measures according to PD-L1 expression level.
Based on a pre-specified interim analysis conducted by the Independent Data Monitoring Committee (IDMC), at 22.1 months of follow up, the median OS was 18.1 months with the combination immunotherapy versus 14.1 months with combination chemotherapy (HR=0.74; P=0.002), suggesting a 26% reduction in the risk of death when treated with OPDIVO® plus YERVOY®. The 12- month OS rates were 68% and 58%, and 2-year OS rates were 41% and 27%, respectively. This OS benefit with combination immunotherapy was observed in both non-epithelioid and epithelioid Malignant Pleural Mesothelioma, regardless of PD-L1 expression.
The median PFS per Blinded Independent Central Review (BICR) was 6.8 months in the combination immunotherapy group, and 7.2 months in the combination chemotherapy group. However, combination immunotherapy resulted in a higher PFS rate at both 12 months and 24 months compared to the combination chemotherapy arm, at 30% versus 16%, and 24% versus 7%, respectively. The confirmed Objective Response Rate was 40% and 43% in the OPDIVO® plus YERVOY® and combination chemotherapy groups, respectively. The median Duration of Response was 11.0 months in the OPDIVO® plus YERVOY® group and 6.7 months in the combination chemotherapy group, with notable differences in response at 12 months (47% versus 26%) and 24 months (32% versus 8%) respectively. The most common adverse reactions in patients receiving the combination of OPDIVO® plus YERVOY® were fatigue, musculoskeletal pain, rash, pruritus, nausea, decreased appetite, diarrhea, cough and dyspnea.
The authors concluded that this is the first positive randomized trial of dual immunotherapy, in the first line treatment of patients with Malignant Pleural Mesothelioma, and the combination of OPDIVO® plus YERVOY® should be considered as the new standard of care.
First-line nivolumab + ipilimumab vs chemotherapy in unresectable malignant pleural mesothelioma: CheckMate 743. Baas P, Scherpereel A, Nowak A, et al. Presented at the International Association for the Study of Lung Cancer 2020 Presidential Symposium; August 8, 2020; Virtual. Abstract 3.

