SUMMARY: The FDA on August 1, 2017 granted regular approval to IDHIFA® (Enasidenib), for the treatment of adult patients with relapsed or refractory Acute Myeloid Leukemia (AML) with an Isocitrate DeHydrogenase-2 (IDH2) mutation, as detected by an FDA-approved test. The American Cancer Society estimates that in 2017, 21,380 new cases of Acute Myeloid Leukemia (AML) will be diagnosed in the United States and 10,590 patients will die of the disease. AML can be considered as a group of heterogeneous diseases with different clinical behavior and outcomes. Cytogenetic analysis has been part of routine evaluation when caring for patients with AML. By predicting resistance to therapy, tumor cytogenetics will stratify patients, based on risk and help manage them accordingly. Even though cytotoxic chemotherapy may lead to long term remission and cure in a minority of patients with favorable cytogenetics, patients with high risk features such as unfavorable cytogenetics, molecular abnormalities, prior myelodysplasia and advanced age, have poor outcomes with conventional chemotherapy alone.
Isocitrate DeHydrogenase (IDH) is a metabolic enzyme that helps generate energy from glucose and other metabolites by catalyzing the conversion of Isocitrate to Alpha-Ketoglutarate. Alpha-ketoglutarate is required to properly regulate DNA and histone methylation, which in turn is important for gene expression and cellular differentiation. IDH mutations lead to aberrant DNA methylation and altered gene expression thereby preventing cellular differentiation, with resulting immature undifferentiated cells. IDH mutations may thus promote leukemogenesis in Acute Myeloid Leukemia and tumorigenesis in solid tumors. There are three isoforms of IDH. IDH1 is mainly found in the cytoplasm, as well as in peroxisomes, whereas IDH2 and IDH3 are found in the mitochondria, and are a part of the Krebs cycle. Approximately 20% of patients with AML, 70% of patients with Low-grade Glioma and secondary Glioblastoma, 50% of patients with Chondrosarcoma, 20% of patients with Intrahepatic cholangiocarcinoma, 30% of patients with Angioimmunoblastic T-cell lymphoma and 8% of patients with Myelodysplastic syndromes/Myeloproliferative neoplasms, are associated with IDH mutations.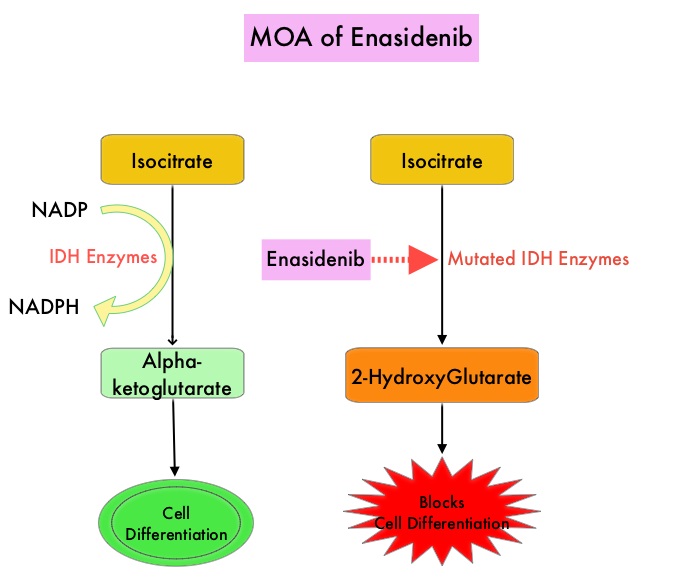
IDHIFA® is an oral, selective, small molecule inhibitor of mutated IDH2 protein. The approval of IDHIFA® was based on an open label, single arm, multicenter, clinical trial that included 199 adults with relapsed or refractory AML, who had an IDH2 mutation as detected by the RealTime IDH2 Assay. Patients received IDHIFA® 100 mg orally daily. The median age was 67 years, the median number of prior therapies was 2 and a third of the patients had unfavorable cytogenetics. Study endpoints included Complete Response (CR) and Complete Response with partial hematologic recovery (CRh) rates, CR/CRh duration, and conversion from transfusion dependence to transfusion independence.
After a median follow up of 6.6 months, 23% of patients experienced CR or CRh lasting a median of 8.2 months, with 19% of patients having a CR lasting a median 8.2 months, and 4% with a CRh lasting a median 9.6 months. The median time to first response was 1.9 months and the median time to best response of CR/CRh was 3.7 months. Of the 157 patients who required transfusions at the initiation of the trial, 34% of the patients no longer required transfusions during at least one 8 week time period on IDHIFA®. Of the 42 patients who did not require transfusions at the start of the study, 76% maintained transfusion independence. The most common toxicities were nausea, vomiting, diarrhea, elevated bilirubin and decreased appetite. Differentiation syndrome occurred in 14% of patients and these patients should be promptly managed, as this could be fatal.
The authors concluded that IDHIFA® is well tolerated and induced lasting Complete Responses in patients who had failed prior AML therapies, with the clinical efficacy related to differentiation of myeloblasts rather than cytotoxicity. This is the first FDA approval for relapsed or refractory AML specifically with an IDH2 mutation. Enasidenib in mutant-IDH2 relapsed or refractory acute myeloid leukemia (R/R AML): Results of a phase I dose-escalation and expansion study. Stein EM, Dinardo CD, Pollyea DA, et al. J Clin Oncol 35, 2017 (suppl; abstr 7004).

