SUMMARY: The American Cancer Society estimates that in the US, about 27,600 new cases of Gastric cancer will be diagnosed in 2020 and about 11,010 people will die of the disease. It is one of the leading causes of cancer-related deaths in the world. Several hereditary syndromes such as Hereditary Diffuse Gastric Cancer (HDGC), Lynch syndrome (Hereditary Nonpolyposis Colorectal Cancer) and Familial Adenomatous Polyposis (FAP) have been associated with a predisposition for stomach cancer. Additionally, one of the strongest risk factor for Gastric adenocarcinoma is infection with Helicobacter pylori (H.pylori), which is a gram-negative, spiral-shaped microaerophilic bacterium.
The Human Epidermal growth factor Receptor (HER) or erbB family of receptors, consist of HER1, HER2, HER3 and HER4. Approximately 15-20% of advanced Gastric and GastroEsophageal (GE) junction cancers, overexpress or have amplification of the HER2 oncogene. These patients often receive first line treatment with a combination of chemotherapy plus anti-HER2 antibody, Trastuzumab, as there is Overall Survival (OS) benefit with this combination regimen. Upon progression, Paclitaxel plus CYRAMZA® (Ramucirumab), an anti-VEGFR-2 antibody is recommended as second-line therapy, regardless of HER2 expression, based on OS and Progression Free Survival (PFS) data for this combination regimen. Trifluridine-tipiracil (LONSURF®) and Immune Checkpoint Inhibitors are treatment options for later lines of therapy and are associated with minimal prolongation in OS. Unlike treatment in metastatic breast cancer, re-treatment with Trastuzumab in combination with various different chemotherapy agents has not shown survival benefit in Gastric cancer. Further, Antibody-Drug Conjugate such as KADCYLA® (ado-trastuzumab emtansine), did not prolong median OS or improve Response Rates compared to chemotherapy, in patients with Gastric cancer who had progressed during or after treatment with Trastuzumab.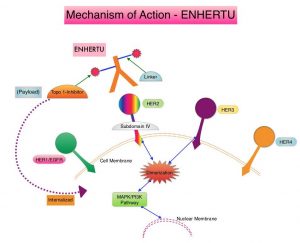
ENHERTU® (Trastuzumab Deruxtecan) is an Antibody-Drug Conjugate (ADC) composed of a humanized monoclonal antibody specifically targeting HER2, with the amino acid sequence similar to Trastuzumab, a cleavable tetrapeptide-based linker, and a potent cytotoxic Topoisomerase I inhibitor as the cytotoxic drug (payload). ENHERTU® has a favorable pharmacokinetic profile and the tetrapeptide-based linker is stable in the plasma and is selectively cleaved by cathepsins that are up-regulated in tumor cells. Unlike KADCYLA®, ENHERTU® has a higher drug-to-antibody ratio (8 versus 4), released payload easily crosses the cell membrane with resulting potent cytotoxic effect on neighboring tumor cells regardless of target expression, and the released cytotoxic agent (payload) has a short half-life, minimizing systemic exposure.
DESTINY-Gastric01 is an open-label, randomized, multicenter, Phase II trial in which ENHERTU® was compared with chemotherapy in patients with HER2-positive advanced Gastric cancer. In this study 187 patients were randomly assigned in a 2:1 ratio to receive either ENHERTU® (N=125) or the treating physician’s choice of Irinotecan or Paclitaxel (N=62). Eligible patients had HER2-expressing advanced Gastric cancer or GastroEsophageal junction adenocarcinoma that had progressed after the receipt of at least two previous systemic therapies, which included a Fluoropyrimidine, a Platinum agent, and Trastuzumab (or approved biosimilar agent). Patients in the ENHERTU® group received the drug at a dose of 6.4 mg/kg as IV infusion every 3 weeks, whereas the physician’s choice group received either Irinotecan monotherapy 150 mg/m2 IV every 2 weeks, or Paclitaxel monotherapy 80 mg/m2 IV on days 1, 8, and 15 every 4 weeks. HER2 levels were documented as high if the score was 3+ on IHC, or 2+ on IHC with positive results on FISH, and documented as low if the score was 2+ on IHC with negative results on FISH, or a score of 1+ on IHC (negative). Treatment was continued until disease progression or unacceptable toxicities. Both treatment groups were well balanced. Approximately 72% of the patients had previously received CYRAMZA® (Ramucirumab), and 86% had received Taxanes. The median time since the last administration of Trastuzumab was 6.2 months. The Primary end point was the Objective Response Rate (ORR), according to Independent Central Review. Secondary end points included Overall Survival (OS), response duration, Progression Free Survival, and safety. The primary cohort consisted of patients with high-level HER2-positive disease, and was the focus of this analysis.
Treatment with ENHERTU® resulted in an ORR of 51%, compared to 14% in the physician’s choice group (P<0.001), according to Independent Central Review. An ORR lasting 4 weeks or more occurred in 43% of patients in the ENHERTU® group, as compared with 12% in the physician’s choice group. More than 80% of patients receiving ENHERTU® had a reduction in tumor size, compared with approximately half the patients receiving physician’s choice of chemotherapy. The median duration of confirmed objective response was 11.3 months in the ENHERTU® group, compared with 3.9 months in the physician’s choice group. Treatment with ENHERTU® resulted in a higher percentage of patients with confirmed disease control (86%), than physician’s choice of chemotherapy (62%). The ORR was higher among those with a HER2 score of 3+ on IHC, than among those with an IHC score of 2+ with positive results on FISH (58% versus 29%).
The Overall Survival was significantly longer in the ENHERTU® group compared to the physician’s choice group (median 12.5 months versus 8.4 months; HR=0.59; P=0.01). The estimated OS at 6 months was 80% in the ENHERTU® group and 66% in the physician’s choice group and at 12 months was 52% and 29%, respectively. In a prespecified subgroup analysis, OS benefit was greater with ENHERTU® compared to physician’s choice of chemotherapy, across most subgroups. The median PFS was 5.6 months in the ENHERTU® group and 3.5 months in the physician’s choice group (HR=0.47). The most common adverse events of Grade 3 or higher were cytopenias. ENHERTU® related Interstitial Lung Disease or pneumonitis was noted in 10% of patients and most events were Grade 1 or 2. Decrease in left ventricular ejection fraction or heart failure was not observed in either treatment groups.
It was concluded that treatment with ENHERTU® resulted in significant improvements in Objective Response Rates and Overall Survival, as compared with standard therapies, among patients with HER2-positive advanced Gastric or GastroEsophageal junction cancer. This benefit was seen even in patients who had disease progression while on Trastuzumab containing regimens.
Trastuzumab Deruxtecan in Previously Treated HER2-Positive Gastric Cancer. Shitara K, Bang Y-J, Iwasa S, et al. for the DESTINY-Gastric01 Investigators. N Engl J Med 2020; 382:2419-2430

