SUMMARY: Multiple Myeloma is a clonal disorder of plasma cells in the bone marrow and the American Cancer Society estimates that in the United States, close to 27,000 new cases will be diagnosed in 2015 and 11,240 will die of the disease. The U.S. Food and Drug Administration (FDA) granted accelerated approval on February 23, 2015 to Panobinostat (FARYDAK®), in combination with VELCADE® (Bortezomib) and Dexamethasone, for the treatment of patients with Multiple Myeloma. The authors in the PANORAMA I trial evaluated the outcomes in previously treated advanced Multiple Myeloma patients, by taking advantage of the synergy between VELCADE®, a proteosome inhibitor and FARYDAK® (Panobinostat), a histone deacetylase (HDAC) inhibitor and treating these patients with a combination of these two agents. HDACs are a family of enzymes that play an important role in the regulation of gene expression.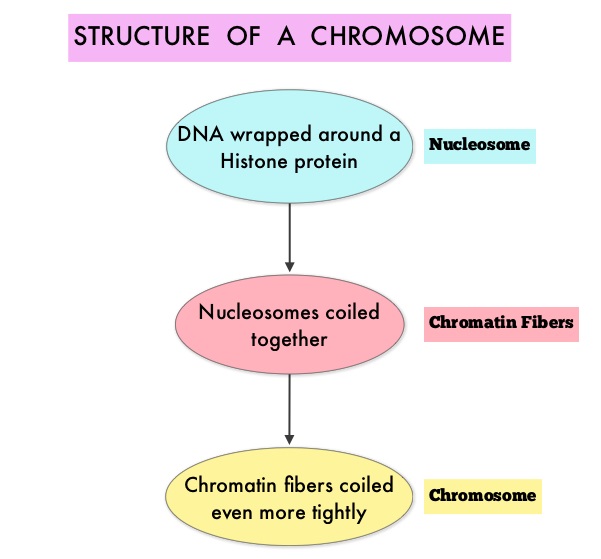 To briefly summarize the structure of a chromosome, individual loops of coiled double-helix DNA wrap around a histone protein to form a nucleosome. Nucleosomes are then coiled together to form chromatin fibers, which looks like beads on a string. The chromatin fibers are coiled even more tightly to form chromosomes. HDAC enzymes catalyze the removal of acetyl groups and regulate the level of acetylation of the histones and non-histone proteins and transcription of several genes. Hypoacetylation of histones has been associated with a condensed chromatin structure that results in the repression of gene transcription, whereas acetylated histones are associated with a more open chromatin structure and activation of gene transcription. HDACs are grouped into four major classes (Class I, II, III and IV) and regulate cell-cycle progression, cell survival, angiogenesis and immunity. The HDAC Class I enzymes are HDAC1, 2, 3 & 8 and are typically found in the nucleus where they are able to repress transcription. The HDAC Class II enzymes include HDAC4, 5, 6, 7, 9 and 10 and are able to move between the cytoplasm and nucleus and function in signal transduction. In Multiple Myeloma, the important enzyme to target is HDAC6.
To briefly summarize the structure of a chromosome, individual loops of coiled double-helix DNA wrap around a histone protein to form a nucleosome. Nucleosomes are then coiled together to form chromatin fibers, which looks like beads on a string. The chromatin fibers are coiled even more tightly to form chromosomes. HDAC enzymes catalyze the removal of acetyl groups and regulate the level of acetylation of the histones and non-histone proteins and transcription of several genes. Hypoacetylation of histones has been associated with a condensed chromatin structure that results in the repression of gene transcription, whereas acetylated histones are associated with a more open chromatin structure and activation of gene transcription. HDACs are grouped into four major classes (Class I, II, III and IV) and regulate cell-cycle progression, cell survival, angiogenesis and immunity. The HDAC Class I enzymes are HDAC1, 2, 3 & 8 and are typically found in the nucleus where they are able to repress transcription. The HDAC Class II enzymes include HDAC4, 5, 6, 7, 9 and 10 and are able to move between the cytoplasm and nucleus and function in signal transduction. In Multiple Myeloma, the important enzyme to target is HDAC6.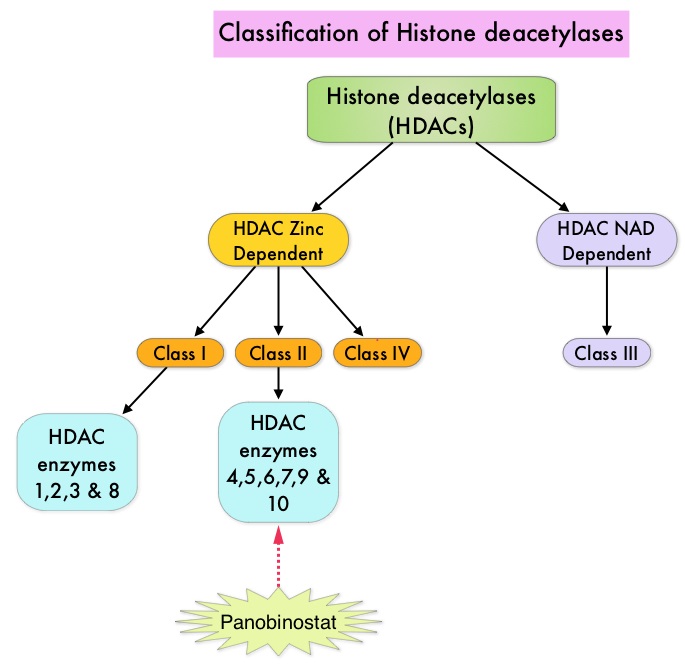 FARYDAK® is an oral, pan-histone deacetylase inhibitor which inhibits cell cycle progression and ultimately results in apoptosis. FARYDAK® inhibits the aggresome pathway of protein degradation which is upregulated when proteosome pathway is inhibited by VELCADE®. Based on preclinical data demonstrating synergy between VELCADE® and FARYDAK® in Myeloma, the PANORAMA 1 trial, enrolled patients with relapsed or refractory Multiple Myeloma who had received one to three prior lines of therapy and were not VELCADE® refractory. In this phase III trial, patients were randomly assigned to receive either FARYDAK® (N=387) or placebo (N=381), each along with IV VELCADE® and oral Dexamethasone. In this study, treatment was given in two 24 week phases. The first 24 week treatment phase was cycles 1 thru 8, where patients received placebo or FARYDAK® 20 mg orally QD 3 times a week for 2 weeks of a 3 week cycle; VELCADE® 1.3 mg/m2 IV twice weekly for 2 weeks of a 3 week cycle and Dexamethasone 20 mg PO on the day of and day after VELCADE®. Patients with clinical benefit (defined as complete response, partial response or stable disease, without significant toxicities) after the first eight cycles could proceed to the second phase of treatment in which FARYDAK® and Dexamethasone administration schedule remained the same but VELCADE® was administered once weekly for 2 weeks of the 3 week cycle. The median age was 63 years, 48% of patients had received at least two lines of therapy and 57% of patients had prior autologous stem cell transplantation and 43% had prior therapy with VELCADE®. The primary endpoint was Progression Free Survival (PFS) and secondary endpoints included Overall Survival (OS), Overall Response Rate (ORR), near Complete/Complete Response (nCR/CR) rate, Duration of Response (DOR), and safety. Among the patients enrolled in the FARYDAK® group (N = 387), 44% completed first phase of treatment and 26% completed the second phase of treatment. After a median follow up of 28 months, the primary end point of the study was met with a 37% decrease in the risk of disease progression in all the FARYDAK® group of patients compared to the placebo group (12 months vs 8.1 months, HR=0.63, P<0.0001). The median PFS was 14.65 months for those in the FARYDAK® group who completed the first phase of treatment and 17.64 months for those who completed the second phase of treatment. With regards to the secondary endpoints in the FARYDAK® vs placebo groups, the ORR was 60.7% vs 54.6% (P=0.87), nCR/CR rate was 27.6% vs 15.7% (P=0.00006), median duration of response was13.1months vs 10.9 months and median time to progression was 12.7 months vs 8.5 months respectively. It was noted that the nCR/CR rate was 52.9% for those patients who completed the second phase of treatment. The most common grade 3/4 adverse events in the FARYDAK® vs placebo arms included thrombocytopenia (67% vs 31%), neutropenia (35% vs 11%), and diarrhea (26% vs 8%) and these toxicities were manageable with dose reduction and supportive care. The authors concluded that a combination of FARYDAK®, VELCADE® and Dexamethasone significantly improves Progression Free Survival in patients with relapsed and refractory Multiple Myeloma, with manageable toxicities. Miguel JS, Hungria VTM , Yoon S, et al. 56th ASH Annual Meeting and Exposition, 2014. Abstract#4742
FARYDAK® is an oral, pan-histone deacetylase inhibitor which inhibits cell cycle progression and ultimately results in apoptosis. FARYDAK® inhibits the aggresome pathway of protein degradation which is upregulated when proteosome pathway is inhibited by VELCADE®. Based on preclinical data demonstrating synergy between VELCADE® and FARYDAK® in Myeloma, the PANORAMA 1 trial, enrolled patients with relapsed or refractory Multiple Myeloma who had received one to three prior lines of therapy and were not VELCADE® refractory. In this phase III trial, patients were randomly assigned to receive either FARYDAK® (N=387) or placebo (N=381), each along with IV VELCADE® and oral Dexamethasone. In this study, treatment was given in two 24 week phases. The first 24 week treatment phase was cycles 1 thru 8, where patients received placebo or FARYDAK® 20 mg orally QD 3 times a week for 2 weeks of a 3 week cycle; VELCADE® 1.3 mg/m2 IV twice weekly for 2 weeks of a 3 week cycle and Dexamethasone 20 mg PO on the day of and day after VELCADE®. Patients with clinical benefit (defined as complete response, partial response or stable disease, without significant toxicities) after the first eight cycles could proceed to the second phase of treatment in which FARYDAK® and Dexamethasone administration schedule remained the same but VELCADE® was administered once weekly for 2 weeks of the 3 week cycle. The median age was 63 years, 48% of patients had received at least two lines of therapy and 57% of patients had prior autologous stem cell transplantation and 43% had prior therapy with VELCADE®. The primary endpoint was Progression Free Survival (PFS) and secondary endpoints included Overall Survival (OS), Overall Response Rate (ORR), near Complete/Complete Response (nCR/CR) rate, Duration of Response (DOR), and safety. Among the patients enrolled in the FARYDAK® group (N = 387), 44% completed first phase of treatment and 26% completed the second phase of treatment. After a median follow up of 28 months, the primary end point of the study was met with a 37% decrease in the risk of disease progression in all the FARYDAK® group of patients compared to the placebo group (12 months vs 8.1 months, HR=0.63, P<0.0001). The median PFS was 14.65 months for those in the FARYDAK® group who completed the first phase of treatment and 17.64 months for those who completed the second phase of treatment. With regards to the secondary endpoints in the FARYDAK® vs placebo groups, the ORR was 60.7% vs 54.6% (P=0.87), nCR/CR rate was 27.6% vs 15.7% (P=0.00006), median duration of response was13.1months vs 10.9 months and median time to progression was 12.7 months vs 8.5 months respectively. It was noted that the nCR/CR rate was 52.9% for those patients who completed the second phase of treatment. The most common grade 3/4 adverse events in the FARYDAK® vs placebo arms included thrombocytopenia (67% vs 31%), neutropenia (35% vs 11%), and diarrhea (26% vs 8%) and these toxicities were manageable with dose reduction and supportive care. The authors concluded that a combination of FARYDAK®, VELCADE® and Dexamethasone significantly improves Progression Free Survival in patients with relapsed and refractory Multiple Myeloma, with manageable toxicities. Miguel JS, Hungria VTM , Yoon S, et al. 56th ASH Annual Meeting and Exposition, 2014. Abstract#4742

