SUMMARY:Chronic Myeloid Leukemia (CML) constitutes approximately 10% of all new cases of leukemia. The American Cancer Society estimates that 5,980 new CML cases will be diagnosed in the United States in 2014 and 810 people will die of the disease. The hallmark of CML, the Philadelphia Chromosome (Chromosome 22), is a result of a reciprocal translocation between chromosomes 9 and 22, wherein the ABL gene from chromosome 9 fuses with the BCR gene on chromosome 22. As a result, the auto inhibitory function of the ABL gene is lost and the BCR-ABL fusion gene is activated resulting in cell proliferation and leukemic transformation of hematopoietic stem cells. The presently available Tyrosine Kinase Inhibitors (TKI’s) approved in the United States including GLEEVEC®, share the same therapeutic target, which is BCR-ABL kinase. Resistance to TKI’s occur as a result of mutations in the BCR-ABL kinase domain or amplification of the BCR-ABL gene. With the availability of newer therapies for CML, monitoring response to treatment is important. This is best accomplished by measuring the amount of residual disease using Reverse Transcription-Polymerase Chain Reaction (RT-PCR).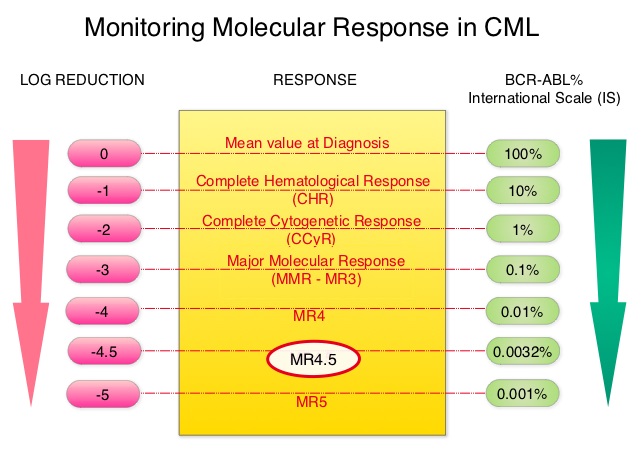 This is expressed using the International Scale (IS) as BCR-ABL%, which is the ratio between BCR-ABL and a control gene. BCR-ABL kinase domain point mutations are detected, using the mutational analysis by Sanger sequencing. Majority of the patients receiving a TKI following diagnosis of CML achieve a Complete Cytogenetic Response (CCyR) within 12 months following commencement of therapy and these patients have a life expectancy similar to that of their healthy counterparts. However, some patients have deeper responses (MR3, MR4, and MR4.5) and it is presumed that this subgroup of patients with CML may stay in unmaintained remission even after treatment discontinuation. Moreover, it is not clear what proportion of patients with CML achieve deeper responses and deeper responses have not been shown to increase survival beyond that associated with CCyR. To address these questions, the authors in this report analyzed the data from the randomized CML – Study IV to characterize the frequency and impact of deep molecular response on survival with different treatment modalities. The study is a five arm trial in which the treatment groups included high dose Imatinib (GLEEVEC® 800 mg/day), GLEEVEC® 400 mg/day, GLEEVEC® 400 mg/day in combination with Interferon alfa (IFN), GLEEVEC® 400 mg/day in combination with Cytarabine, and GLEEVEC® 400 mg/day after IFN failure. The analysis included a total of 1538 patients and the principal objective of CML – Study IV was to determine the impact of MMR (Major Molecular Response) on survival, remission rates and survival probabilities. After a median follow up of 67.5 months, 5 year overall survival was 90%, 8 year overall survival was 86% and 5 year PFS was 87.5%. The cumulative rate of MR4.5, irrespective of treatment group (defined as 4.5 or more log reduction in BCR-ABL transcripts), was 66% at 8 years and 70% at 9 years and the median time to reaching MR4.5 was 4.9 years. High dose GLEEVEC® therapy and early Major Molecular Remission predicted deep molecular response (MR4.5). High dose GLEEVEC® resulted in a more rapid MR4.5 than with GLEEVEC® 400 mg/day (P = .016). Finally, this analysis showed that a confirmed MR4.5 at 4 years predicted significantly higher 8 year overall survival probability compared to CCyR (Complete Cytogenetic response: IS 1%) or MMR (major molecular response: IS 0.1%) – 92% versus 83%, P=0.047. The authors concluded that deep molecular response (MR4.5) is a new molecular predictor of long term survival in CML patients and is achieved in a majority of patients treated with GLEEVEC®, and is achieved more rapidly with optimized high-dose GLEEVEC®. The authors further pointed out that none of the patients with confirmed MR4.5 had disease progression and this may therefore provide a therapeutic rationale for discontinuing treatment in this subset of patients with CML. These findings may also justify the use of more effective second generation TKI’s to induce early and deep molecular responses. Hehlmann R, Müller MC, Lauseker M, et al. J Clin Oncol 2014;32:415-423
This is expressed using the International Scale (IS) as BCR-ABL%, which is the ratio between BCR-ABL and a control gene. BCR-ABL kinase domain point mutations are detected, using the mutational analysis by Sanger sequencing. Majority of the patients receiving a TKI following diagnosis of CML achieve a Complete Cytogenetic Response (CCyR) within 12 months following commencement of therapy and these patients have a life expectancy similar to that of their healthy counterparts. However, some patients have deeper responses (MR3, MR4, and MR4.5) and it is presumed that this subgroup of patients with CML may stay in unmaintained remission even after treatment discontinuation. Moreover, it is not clear what proportion of patients with CML achieve deeper responses and deeper responses have not been shown to increase survival beyond that associated with CCyR. To address these questions, the authors in this report analyzed the data from the randomized CML – Study IV to characterize the frequency and impact of deep molecular response on survival with different treatment modalities. The study is a five arm trial in which the treatment groups included high dose Imatinib (GLEEVEC® 800 mg/day), GLEEVEC® 400 mg/day, GLEEVEC® 400 mg/day in combination with Interferon alfa (IFN), GLEEVEC® 400 mg/day in combination with Cytarabine, and GLEEVEC® 400 mg/day after IFN failure. The analysis included a total of 1538 patients and the principal objective of CML – Study IV was to determine the impact of MMR (Major Molecular Response) on survival, remission rates and survival probabilities. After a median follow up of 67.5 months, 5 year overall survival was 90%, 8 year overall survival was 86% and 5 year PFS was 87.5%. The cumulative rate of MR4.5, irrespective of treatment group (defined as 4.5 or more log reduction in BCR-ABL transcripts), was 66% at 8 years and 70% at 9 years and the median time to reaching MR4.5 was 4.9 years. High dose GLEEVEC® therapy and early Major Molecular Remission predicted deep molecular response (MR4.5). High dose GLEEVEC® resulted in a more rapid MR4.5 than with GLEEVEC® 400 mg/day (P = .016). Finally, this analysis showed that a confirmed MR4.5 at 4 years predicted significantly higher 8 year overall survival probability compared to CCyR (Complete Cytogenetic response: IS 1%) or MMR (major molecular response: IS 0.1%) – 92% versus 83%, P=0.047. The authors concluded that deep molecular response (MR4.5) is a new molecular predictor of long term survival in CML patients and is achieved in a majority of patients treated with GLEEVEC®, and is achieved more rapidly with optimized high-dose GLEEVEC®. The authors further pointed out that none of the patients with confirmed MR4.5 had disease progression and this may therefore provide a therapeutic rationale for discontinuing treatment in this subset of patients with CML. These findings may also justify the use of more effective second generation TKI’s to induce early and deep molecular responses. Hehlmann R, Müller MC, Lauseker M, et al. J Clin Oncol 2014;32:415-423

