SUMMARY: Multiple Myeloma is a clonal disorder of plasma cells in the bone marrow and the American Cancer Society estimates that in the United States, 32,110 new cases will be diagnosed in 2019 and 12,960 patients are expected to die of the disease. Multiple Myeloma (MM) in 2019 remains an incurable disease. The therapeutic goal therefore is to improve Progression Free Survival (PFS) and Overall Survival (OS). Multiple Myeloma is a disease of the elderly, with a median age at diagnosis of 69 years and characterized by intrinsic clonal heterogeneity. Almost all patients eventually will relapse, and patients with a high-risk cytogenetic profile or refractory disease have the worst outcomes. The median survival for patients with myeloma is over 10 years.
Elderly patients with myeloma in the US are often treated with a combination of REVLIMID® (Lenalidomide) and Dexamethasone, whereas Melphalan, Prednisone, and Thalidomide (MPT) and VELCADE® (Bortezomib), Melphalan and Prednisone (VMP) are the most widely used regimens outside the US. These regimens are associated with a PFS of 18-24 months and an OS of 4-5 years. For patients with newly diagnosed multiple myeloma who are ineligible for ASCT, treatment with VMP regimen has been a standard effective regimen, based on the VISTA (Velcade as Initial Standard Therapy in Multiple Myeloma: Assessment with Melphalan and Prednisone) trial.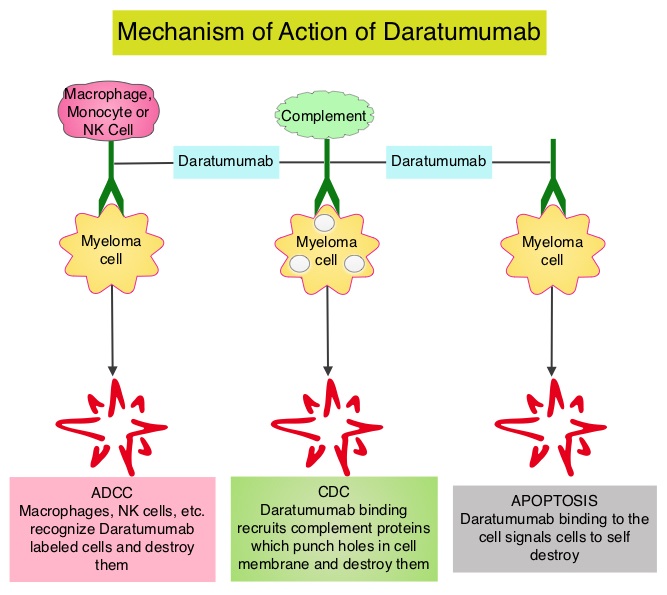
DARZALEX® (Daratumumab) is a human IgG1 antibody that targets CD38, a transmembrane glycoprotein abundantly expressed on malignant plasma cells and with low levels of expression on normal lymphoid and myeloid cells. DARZALEX® exerts its cytotoxic effect on myeloma cells by multiple mechanisms, including Antibody Dependent Cellular Cytotoxicity (ADCC), Complement Dependent Cytotoxicity (CDC) and direct Apoptosis. Additionally, DARZALEX® may have a role in immunomodulation, by depleting CD38-positive regulator immune suppressor cells, and thereby expanding T cells, in patients responding to therapy.
ALCYONE is a multicenter, randomized, open-label, active-controlled, Phase III trial in which DARZALEX® given along with VELCADE®, Melphalan and Prednisone (D-VMP regimen) was compared with VMP alone (control group), in patients with newly diagnosed multiple myeloma, who were ineligible for Autologous Stem Cell Transplantation (ASCT). Of the 706 enrolled patients, 350 were assigned to the DARZALEX® group and 356 to the control group. The median age was 71 yrs. All the patients received up to nine 6 week cycles of VELCADE® 1.3 mg/m2 SQ, twice weekly on weeks 1, 2, 4, and 5 of cycle 1 and once weekly on weeks 1, 2, 4, and 5 of cycles 2-9), Melphalan 9 mg/m2 orally, once daily on days 1-4 of each cycle, and Prednisone 60 mg/m2 once daily on days 1-4 of each cycle. In the study group, patients received DARZALEX® 16 mg/kg IV administered with Dexamethasone 20 mg oral or IV (to manage infusion reactions), once weekly for a total of 6 doses, every 3 weeks for a total of 16 doses and every 4 weeks thereafter until disease progression or unacceptable toxicity. The Primary end point was Progression Free Survival (PFS). Secondary end points included Overall Response Rate (ORR), rates of Very Good Partial Response (VGPR), Complete Response (CR) rate, Minimal Residual Disease (MRD) negativity and Overall Survival (OS). The FDA in 2018 approved DARZALEX® in combination with VELCADE® (Bortezomib), a proteasome inhibitor, Melphalan, an alkylating agent and Prednisone VMP regimen), for the treatment of patients with newly diagnosed multiple myeloma who are ineligible for Autologous Stem Cell Transplant (ASCT), based on the Progression Free Survival (PFS) benefit at 16.5 months, noted during the primary analysis of the ALCYONE study. The authors herein presented outcomes after more than 36 months of follow-up from the ALCYONE study, including analysis of Overall Survival (OS) from a prespecified interim analysis.
In this updated analysis, treatment with D-VMP continued to demonstrate a twofold greater median PFS at 36.4 months versus 19.3 months with VMP, after a median follow-up of 41months (HR=0.42; P<0.0001). Patients assigned to D-VMP also had significantly prolonged PFS on subsequent therapy (PFS-2). Median PFS-2 was not reached with D-VMP versus 42.3 months with VMP (HR=0.55; P<0.0001), representing a 45% reduction in the risk for progression or death. The median time to subsequent therapy had yet to be reached in the D-VMP group versus 25.9 months for the VMP group. The Overall Response Rate was 91% in the D-VMP group as compared with 74% in the control group and the rate of Complete Response or better (including stringent CR) was 46%, versus 24.4% in the control group. The MRD-negative rate (at a threshold of 1 tumor cell per 105 white cells) was 28% with D-VMP and 7% with VMP and D-VMP also led to higher rates of sustained MRD negativity. MRD negativity for 12 or more months was associated with improved PFS. The estimated 36 month OS rate was 78% with D-VMP versus 68% with VMP, with a significant benefit for OS observed for D-VMP versus VMP alone (HR=0.60; P=0.0003). This represented a 40% reduction in the risk of death, in favor of D-VMP.
The authors concluded that for the first time, this study demonstrated that the addition of DARZALEX® to VMP significantly prolonged Overall Survival in patients with transplant-ineligible newly diagnosed Multiple Myeloma, with a 40% reduction in the risk of death, when compared with VMP alone. They added that these findings, together with the Phase 3 MAIA study (DARZALEX® plus Lenalidomide and Dexamethasone versus Lenalidomide plus Dexamethasone), continue to support the addition of DARZALEX® to frontline treatment regimens for patients with Multiple Myeloma. Daratumumab Plus Bortezomib, Melphalan, and Prednisone Versus Bortezomib, Melphalan, and Prednisone in Patients with Transplant-Ineligible Newly Diagnosed Multiple Myeloma: Overall Survival in Alcyone. Mateos MV, Cavo M, Bladé J, et al. Presented at: 2019 ASH Annual Meeting; December 7-10, 2019; Orlando, FL. Abstract 859.

