SUMMARY: Circulating tumor cells (CTCs) are epithelial cells that are shed into the circulation from a primary or metastatic tumor. After being shed, CTCs can remain in the circulation or undergo apoptosis. Evaluation of CTCs during the course of disease and treatment has prognostic value. Because of the very low concentrations of CTCs (1 CTC in the background of millions of normal hematopoietic cells) in the peripheral blood, different technologies have been developed that will allow enrichment and detection of these CTCs.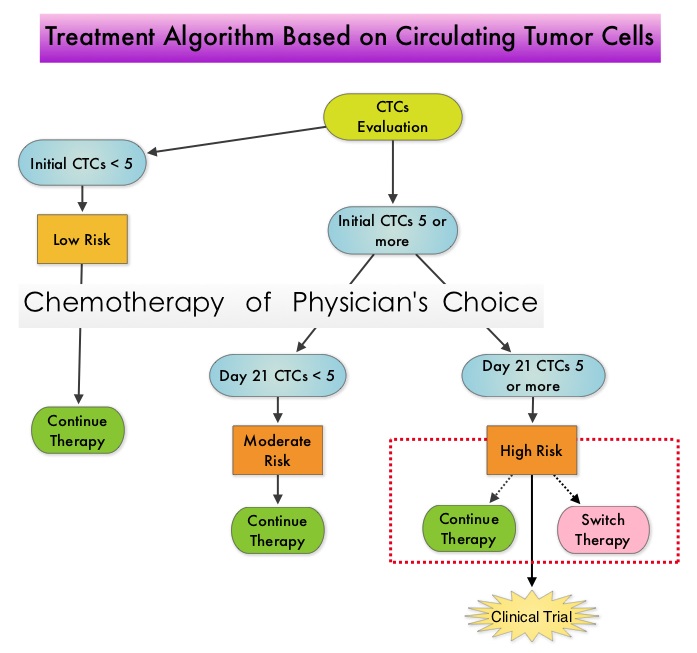 One such technology is the CellSearch® system which is the first FDA-approved test for CTC assessment, in the peripheral blood of Metastatic Breast Cancer (MBC) patients. This automated system is able to enrich the peripheral blood sample with CTCs and the cells then are fluorescently stained for CytoKeratins (CK8,18 and 19), Common Leukocyte Antigen (CD45) and a nuclear dye (DAPI). CTCs are identified when they are CK and DAPI positive and CD45 negative. In essence, CTC assessment, is a real time, peripheral blood evaluation (“Liquid Biopsy”) in MBC patients. Previously published studies have concluded that in patients with MBC, increased levels of CTCs prior to administration of a new therapy was associated with poor outcomes and failure of CTCs to drop to below 5 CTCs per 7.5 mL of peripheral blood at 3- 5 weeks after systemic therapy initiation, predicted worse Progression Free Survival (PFS) and Overall Survival (OS), compared to those who did not have increased CTCs at baseline or had increased CTCs at baseline and but not at 3-5 weeks after therapy. With this background, a randomized study was conducted to assess whether changing treatment after one cycle of first line chemotherapy, in those with a persistent increase in CTCs, improved OS. Evaluable patients were initially divided into two groups – Group A (N=276) included patients who did not have increased CTCs at baseline and Group B (N=288) included patients who had 5 or more CTCs per 7.5 mL of peripheral blood. Eligible patients were chemotherapy naïve for MBC and were treated with single agent chemotherapy. The choice of chemotherapy was at the discretion of the attending physician. Patients in Group A remained on initial therapy until disease progression whereas patients in Group B had CTC evaluation at Day 22 and those with decreased CTCs remained on the initial therapy (N=165). Patients who had persistently increased CTCs at Day 22 (N=123), were then randomly assigned to either continue the initial therapy (Group C1) or switch to a different chemotherapy regimen (Group C2). The median Overall Survival for Groups A, B, and C (C1 and C2 combined) were 35 months, 23 months, and 13 months, respectively (P <0.001). There was no difference in median Overall Survival between Groups C1 and C2 (10.7 and 12.5 months, respectively (P = 0.98). The authors concluded that CTCs in patients with Metastatic Breast Cancer receiving first line chemotherapy has significant prognostic value and changing to a different chemotherapy regimen based on persistently increased CTCs after 3 weeks of first line chemotherapy, had no impact in prolonging Overall Survival. This group of patients (C1 and C2) should be encouraged to enroll in clinical trials as standard chemotherapy may not be as effective. CTC count can prognosticate Progression Free Survival and Overall Survival early in the treatment course thereby allowing customized care. Further, CTC enumeration, unlike mucin based serum biomarkers such as CEA and CA15-3, better correlates with clinical and pathological characteristics of the disease. Smerage JB, Barlow WE, Hortobagyi GN, et al. DOI: 10.1200/JCO.2014.56.2561
One such technology is the CellSearch® system which is the first FDA-approved test for CTC assessment, in the peripheral blood of Metastatic Breast Cancer (MBC) patients. This automated system is able to enrich the peripheral blood sample with CTCs and the cells then are fluorescently stained for CytoKeratins (CK8,18 and 19), Common Leukocyte Antigen (CD45) and a nuclear dye (DAPI). CTCs are identified when they are CK and DAPI positive and CD45 negative. In essence, CTC assessment, is a real time, peripheral blood evaluation (“Liquid Biopsy”) in MBC patients. Previously published studies have concluded that in patients with MBC, increased levels of CTCs prior to administration of a new therapy was associated with poor outcomes and failure of CTCs to drop to below 5 CTCs per 7.5 mL of peripheral blood at 3- 5 weeks after systemic therapy initiation, predicted worse Progression Free Survival (PFS) and Overall Survival (OS), compared to those who did not have increased CTCs at baseline or had increased CTCs at baseline and but not at 3-5 weeks after therapy. With this background, a randomized study was conducted to assess whether changing treatment after one cycle of first line chemotherapy, in those with a persistent increase in CTCs, improved OS. Evaluable patients were initially divided into two groups – Group A (N=276) included patients who did not have increased CTCs at baseline and Group B (N=288) included patients who had 5 or more CTCs per 7.5 mL of peripheral blood. Eligible patients were chemotherapy naïve for MBC and were treated with single agent chemotherapy. The choice of chemotherapy was at the discretion of the attending physician. Patients in Group A remained on initial therapy until disease progression whereas patients in Group B had CTC evaluation at Day 22 and those with decreased CTCs remained on the initial therapy (N=165). Patients who had persistently increased CTCs at Day 22 (N=123), were then randomly assigned to either continue the initial therapy (Group C1) or switch to a different chemotherapy regimen (Group C2). The median Overall Survival for Groups A, B, and C (C1 and C2 combined) were 35 months, 23 months, and 13 months, respectively (P <0.001). There was no difference in median Overall Survival between Groups C1 and C2 (10.7 and 12.5 months, respectively (P = 0.98). The authors concluded that CTCs in patients with Metastatic Breast Cancer receiving first line chemotherapy has significant prognostic value and changing to a different chemotherapy regimen based on persistently increased CTCs after 3 weeks of first line chemotherapy, had no impact in prolonging Overall Survival. This group of patients (C1 and C2) should be encouraged to enroll in clinical trials as standard chemotherapy may not be as effective. CTC count can prognosticate Progression Free Survival and Overall Survival early in the treatment course thereby allowing customized care. Further, CTC enumeration, unlike mucin based serum biomarkers such as CEA and CA15-3, better correlates with clinical and pathological characteristics of the disease. Smerage JB, Barlow WE, Hortobagyi GN, et al. DOI: 10.1200/JCO.2014.56.2561

