SUMMARY: The American Cancer Society estimates that for 2021, about 21,250 new cases of Chronic Lymphocytic Leukemia (CLL) will be diagnosed in the US and 4320 patients will die of the disease. CLL accounts for about one-quarter of the new cases of leukemia. The average age of patients diagnosed with CLL is around 70 years, and is rarely seen in people under age 40, and is extremely rare in children.
Bruton’s Tyrosine Kinase (BTK) is a member of the Tec family of kinases, downstream of the B-cell receptor and is predominantly expressed in B-cells. It is a mediator of B-cell receptor signaling in normal and transformed B-cells. Ibrutinib (IMBRUVICA®) is an oral, irreversible inhibitor of BTK and inhibits cell proliferation and promotes programmed cell death (Apoptosis) by blocking B-cell activation and signaling. Ibrutinib demonstrated survival benefits when compared to chemoimmunotherapy both in previously untreated (RESONATE-2), as well as relapsed (RESONATE) CLL patients. However, toxicities leading to Ibrutinib discontinuation occurred in a significant number of patients, and Atrial Fibrillation was noted in 11-16% of patients and hypertension rates were between 20-26%.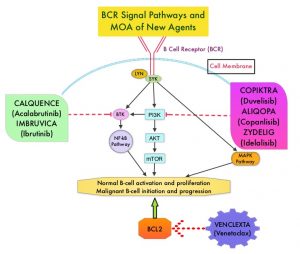
Acalabrutinib (CALQUENCE®) is a highly selective, next-generation, oral, covalent, irreversible Bruton Tyrosine Kinase (BTK) inhibitor with minimal activity against other kinases. Acalabrutinib has a shorter plasma half-life and inhibits cell proliferation and promotes programmed cell death (Apoptosis) by blocking B-cell activation and signaling. Acalabrutinib demonstrated superior Progression Free Survival (PFS) versus chemoimmunotherapy in patients with previously untreated (ELEVATE-TN), as well as Relapsed or Refractory (ASCEND) CLL. Acalabrutinib was better tolerated with lower rates of treatment discontinuation due to adverse events, and also demonstrated efficacy and tolerability in Ibrutinib-intolerant patients with CLL.
ELEVATE-RR is a prospective, randomized, multicenter, open-label, noninferiority, Phase III study, conducted to compare the efficacy and safety of Acalabrutinib with Ibrutinib, in patients with previously treated CLL, and to test the hypothesis that Acalabrutinib was noninferior to Ibrutinib in PFS, with improved tolerability. This trial included 533 previously treated patients with CLL who were randomly assigned 1:1 to receive Acalabrutinib 100 mg orally twice daily (N=268) or Ibrutinib 420 mg orally once daily (N=265). Treatment was continued until disease progression or unacceptable toxicity, and crossover between treatment groups was not permitted. Enrolled patients had centrally confirmed del(17)(p13.1) or del(11)(q22.3) and patients were stratified based on cytogenetics, ECOG Performance Status and number of prior therapies. Patients with significant cardiovascular disease, concomitant vitamin K antagonist treatment, prior BTK or BCL2 inhibitor treatment, or those requiring treatment with Proton-Pump Inhibitors were excluded. The Primary end point was Independent Review Committee-assessed noninferiority of Progression Free Survival (PFS). Secondary end points included incidences of any grade Atrial Fibrillation, Grade 3 or higher infections, Richter transformation and Overall Survival (OS)
After a median follow-up of 40.9 months, Acalabrutinib was determined to be noninferior to Ibrutinib with a median PFS of 38.4 months in both arms (HR=1.00), thus meeting the noninferiority criterion. Any-grade Atrial Fibrillation/Atrial Flutter incidence was significantly lower with Acalabrutinib compared to Ibrutinib (9.4% versus 16.0%; P=0.02). Bleeding events were less frequent with Acalabrutinib (38%) versus Ibrutinib (51.3%). The median Overall Survival was not reached in either treatment groups. Treatment was discontinued due to adverse events in 14.7% of Acalabrutinib-treated patients and 21.3% of Ibrutinib-treated patients.
The authors concluded that this is the first direct comparison of Ibrutinib with Acalabrutinib in CLL and Acalabrutinib demonstrated noninferior PFS and provides improved safety, with fewer Atrial Fibrillation events and discontinuations because of adverse events, when compared to Ibrutinib.
Acalabrutinib Versus Ibrutinib in Previously Treated Chronic Lymphocytic Leukemia: Results of the First Randomized Phase III Trial. Byrd JC, Hillmen P, Ghia P, et al. DOI: 10.1200/JCO.21.01210 Journal of Clinical Oncology. Published online July 26, 2021.

