SUMMARY: The American Cancer Society estimates that about 63,990 new cases of kidney cancer will be diagnosed in the United States in 2017 and about 14,400 patients will die from this disease. The VHL (Von Hippel-Lindau) protein is a tumor suppressor gene which is frequently mutated and inactivated in approximately 90% of clear cell Renal Cell Carcinomas (RCC). The VHL gene under normal conditions binds to Hypoxia-Inducible Factor (HIF-1 alpha) and facilitates degradation of this factor. Under hypoxic conditions and in patients having biallelic loss of function and mutation of VHL genes, HIF-1alpha is not degraded. Build up of HIF-1 alpha results in increased angiogenesis, increased tumor cell proliferation and survival, as well as metastasis.
SUTENT® (Sunitinib) is the standard first-line intervention for treatment naïve patients with advanced Renal Cell Carcinoma. VOTRIENT® (Pazopanib) another VEGFR-targeted therapy, is an alternative choice, as it was found to be non-inferior to SUTENT® in the COMPARZ trial. CABOMETYX® (Cabozantinib) is an oral, small-molecule Tyrosine Kinase Inhibitor (TKI) but, unlike SUTENT® which targets the Vascular Endothelial Growth Factor Receptors (VEGFR), CABOMETYX® additionally inhibits the action of tyrosine kinases MET and AXL. Both MET and AXL are up-regulated in Renal Cell Carcinoma as a consequence of VHL inactivation and increased expression of MET and AXL is associated with tumor progression and development of resistance to VEGFR inhibitors. CABOMETYX® was approved by the FDA in 2016 for the treatment of advanced Renal Cell Carcinoma (RCC), in patients who have received prior anti-angiogenic therapy.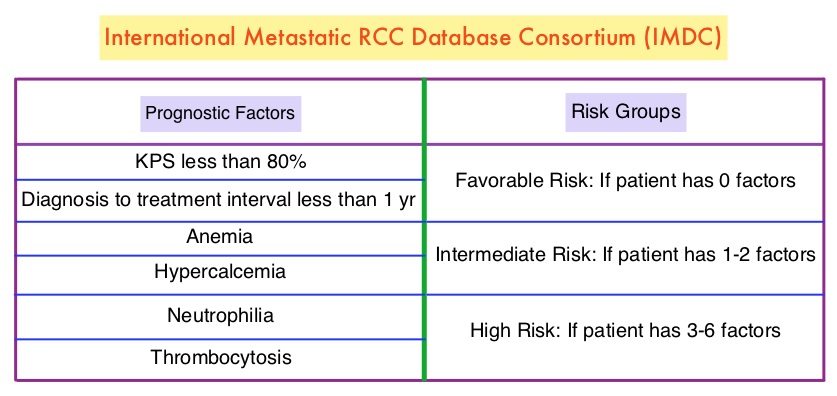
The Alliance for Clinical Trials in Oncology reported the results of a randomized, multicenter, open-label phase II CABOSUN trial, which compared CABOMETYX® with standard-of-care SUTENT®, in IMDC intermediate and poor-risk untreated patients with locally advanced or metastatic clear-cell RCC. The study population had a high rate of bone metastases, a known negative prognostic factor in RCC. This study enrolled 157 patients who were randomized in a 1:1 ratio to receive CABOMETYX® 60 mg orally daily (N=79) or SUTENT® 50 mg orally 4 weeks on, 2 weeks off (N=78). A treatment cycle was defined as 6 weeks in both study groups and treatment was continued until disease progression or intolerance to therapy. Patients were stratified by IMDC risk category (intermediate or poor) and presence of bone metastases. Crossover between treatment groups was not allowed. The median age was 63 years and 81% of the enrolled patients were classified as IMDC intermediate risk and 19% as poor risk, 36% of patients had bone metastases and 75% of the patients had prior nephrectomy. The Primary end point was Progression Free Survival (PFS) and Secondary end points included Objective Response Rate (ORR), Overall Survival (OS) and safety.
It was noted that the treatment with CABOMETYX® significantly increased median PFS compared with SUTENT® (8.2 versus 5.6 months) and was associated with a 34% reduction in rate of disease progression or death (HR=0.66; P=0.012). The ORR was 46% in the CABOMETYXreg; group compared with 18% in the SUTENT® group. The median OS with CABOMETYX® was 30.3 months versus 21.8 months with SUTENT® (HR=0.80), with this preliminary data showing a 20% decrease in the risk of death with CABOMETYX®. Grade 3 or 4 adverse events were similar in both treatment groups with more hypertension and Palmar-Plantar Erythrodysesthesia in the CABOMETYX® group whereas the SUTENT® group experienced more fatigue and hematologic toxicities. Discontinuation rates related to adverse events were similar in both treatment groups.
The authors concluded that CABOMETYX® significantly improved PFS and ORR compared to SUTENT®, in the initial treatment of patients with intermediate or poor-risk clear cell metastatic Renal Cell Carcinoma. CABOMETYX® is the first agent to demonstrate clinical superiority over SUTENT®, which has been the established standard of care for more than 10 years. The authors attributed the superiority of CABOMETYX® over SUTENT® due to its mechanism of action, which targets MET and AXL, in addition to VEGFR. Cabozantinib Versus Sunitinib As Initial Targeted Therapy for Patients With Metastatic Renal Cell Carcinoma of Poor or Intermediate Risk: The Alliance A031203 CABOSUN Trial. Choueiri TK, Halabi S, Sanford BL, et al. DOI: 10.1200/JCO.2016.70.7398 Journal of Clinical Oncology 35, no. 6 (February 2017) 591-597.

