SUMMARY:Breast cancer is the most common cancer among women in the US and about 1 in 8 women (12%) will develop invasive breast cancer during their lifetime. Approximately, 231,840 new cases of invasive breast cancer will be diagnosed in 2015 and over 40,000 women will die of the disease. Taxanes, which include TAXOL® (Paclitaxel) and TAXOTERE® (Docetaxel) have an important role in the treatment of breast cancer and have been shown in numerous clinical studies to improve survival in patients with early-stage breast cancer and select group of patients with metastatic breast cancer.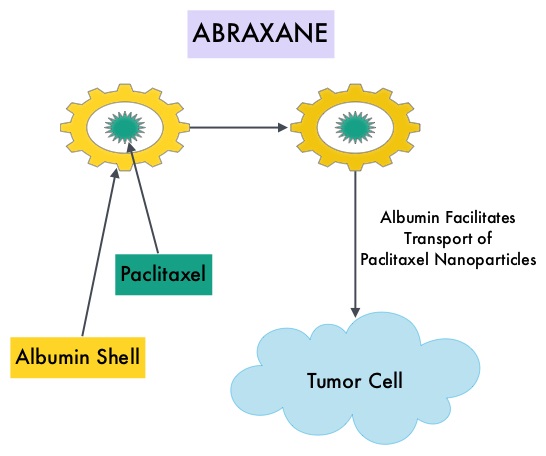 ABRAXANE® (Nab-paclitaxel) is a solvent free, albumin-encapsulated nanoparticle formulation of the taxane, Paclitaxel. By virtue of its formulation, unlike TAXOL®, hypersensitivity reactions are uncommon with ABRAXANE® and can therefore be rapidly infused and premedications are not needed. Further, higher tumor drug concentrations are achieved with ABRAXANE® compared to conventional Paclitaxel (TAXOL®). Previously published studies have shown that ABRAXANE® is superior to TAXOL® in metastatic breast cancer. GeparSepto trial is a randomized phase III study in which weekly ABRAXANE® was compared head to head with weekly TAXOL® in a neoadjuvant setting. This study enrolled 1204 treatment naïve, high risk patients, with clinical T2-T4d invasive breast carcinoma. Eligible patients included those with unilateral, bilateral, operable or inoperable breast cancer. The median age was 49 years, median tumor size was 3 cm, 23% of the patients had triple-negative disease and 33% had HER-2 positive tumors. Patients were randomized 1:1 to receive either ABRAXANE® 125 mg/m2 IV or TAXOL® 80 mg/m2 IV, weekly for 12 weeks followed by 4 cycles of Epirubicin 90 mg/m2 IV and Cyclophosphamide 600 mg/m2 IV. Patients with HER-2 positive tumors also received HERCEPTIN® (Trastuzumab) and PERJETA® (Pertuzumab). The primary endpoint was pathologic Complete Response (pCR), defined as absence of microscopic residual invasive or noninvasive viable tumor cells in all resected specimens of the breast and axilla. This endpoint was chosen because pathologic Complete Response serves as a surrogate marker for long-term efficacy and outcomes. In this trial, the researchers noted a pathologic Complete Response rate of 38% with ABRAXANE® compared to 29% with TAXOL® (P<0.01). On subgroup analysis, this benefit was even more evident in patients with triple negative breast cancer (N=275), with a pCR rate of 48.2% in the ABRAXANE® group compared with 25.7% in the TAXOL® group (P <0.001). The incidence of peripheral neuropathy was higher in the ABRAXANE® group compared to TAXOL® group and this was attributed to higher weekly doses of ABRAXANE® administered. It is felt that a lower dose of weekly ABRAXANE® (100 mg/m2) would result in a decrease in the incidence of peripheral neuropathy, without compromising efficacy. The authors concluded that ABRAXANE® is superior to TAXOL® in early stage, high risk patients with breast cancer and this benefit is even more evident in those patients with triple negative disease, which comprises about 15% of all breast cancers. Untch M, Jackisch C, Schneeweiss A, et al. A randomized phase III trial comparing neoadjuvant chemotherapy with weekly nanoparticle-based paclitaxel with solvent-based paclitaxel followed by anthracyline/cyclophosphamide for patients with early breast cancer (GeparSepto); GBG 69. Paper presented at: 2014 San Antonio Breast Cancer Symposium; December 9-13, 2014; San Antonio, TX. Abstract S2-07.
ABRAXANE® (Nab-paclitaxel) is a solvent free, albumin-encapsulated nanoparticle formulation of the taxane, Paclitaxel. By virtue of its formulation, unlike TAXOL®, hypersensitivity reactions are uncommon with ABRAXANE® and can therefore be rapidly infused and premedications are not needed. Further, higher tumor drug concentrations are achieved with ABRAXANE® compared to conventional Paclitaxel (TAXOL®). Previously published studies have shown that ABRAXANE® is superior to TAXOL® in metastatic breast cancer. GeparSepto trial is a randomized phase III study in which weekly ABRAXANE® was compared head to head with weekly TAXOL® in a neoadjuvant setting. This study enrolled 1204 treatment naïve, high risk patients, with clinical T2-T4d invasive breast carcinoma. Eligible patients included those with unilateral, bilateral, operable or inoperable breast cancer. The median age was 49 years, median tumor size was 3 cm, 23% of the patients had triple-negative disease and 33% had HER-2 positive tumors. Patients were randomized 1:1 to receive either ABRAXANE® 125 mg/m2 IV or TAXOL® 80 mg/m2 IV, weekly for 12 weeks followed by 4 cycles of Epirubicin 90 mg/m2 IV and Cyclophosphamide 600 mg/m2 IV. Patients with HER-2 positive tumors also received HERCEPTIN® (Trastuzumab) and PERJETA® (Pertuzumab). The primary endpoint was pathologic Complete Response (pCR), defined as absence of microscopic residual invasive or noninvasive viable tumor cells in all resected specimens of the breast and axilla. This endpoint was chosen because pathologic Complete Response serves as a surrogate marker for long-term efficacy and outcomes. In this trial, the researchers noted a pathologic Complete Response rate of 38% with ABRAXANE® compared to 29% with TAXOL® (P<0.01). On subgroup analysis, this benefit was even more evident in patients with triple negative breast cancer (N=275), with a pCR rate of 48.2% in the ABRAXANE® group compared with 25.7% in the TAXOL® group (P <0.001). The incidence of peripheral neuropathy was higher in the ABRAXANE® group compared to TAXOL® group and this was attributed to higher weekly doses of ABRAXANE® administered. It is felt that a lower dose of weekly ABRAXANE® (100 mg/m2) would result in a decrease in the incidence of peripheral neuropathy, without compromising efficacy. The authors concluded that ABRAXANE® is superior to TAXOL® in early stage, high risk patients with breast cancer and this benefit is even more evident in those patients with triple negative disease, which comprises about 15% of all breast cancers. Untch M, Jackisch C, Schneeweiss A, et al. A randomized phase III trial comparing neoadjuvant chemotherapy with weekly nanoparticle-based paclitaxel with solvent-based paclitaxel followed by anthracyline/cyclophosphamide for patients with early breast cancer (GeparSepto); GBG 69. Paper presented at: 2014 San Antonio Breast Cancer Symposium; December 9-13, 2014; San Antonio, TX. Abstract S2-07.

