SUMMARY: The American Cancer Society estimates that for 2018, about 20,940 new cases of Chronic Lymphocytic Leukemia (CLL) will be diagnosed in the US and 4,510 patients will die of the disease. CLL accounts for about 25% of the new cases of leukemia and the average age at the time of diagnosis is around 71 years. B-cell CLL is the most common type of leukemia in adults.
The National Cancer Institute sponsored International Workshop on CLL, issued an update to its consensus guidelines originally published in 2008, prompted by the recent advances in the biology and treatment of patients with CLL. The goal of the updated guidelines is to integrate these new findings into clinical practice and CLL clinical trials.
The following are the major changes or additions reflected in the guidelines
Molecular Genetics
Patients with 17p deletion and TP53 mutations have inferior outcomes and have disease, resistant to standard chemotherapy regimens. These genetic abnormalities can also be acquired over the course of the disease. Patients with CLL should therefore be evaluated for these genetic abnormalities at the time of initial diagnosis and prior to any subsequent line of treatment. These patients have better outcomes when treated with non-chemotherapeutic agents, such as Bruton’s Tyrosine Kinase (BTK) inhibitors, Phosphatidylinositol 3-Kinase (PI3K) inhibitors and BCL2 inhibitors. Even though mutations in NOTCH1 or SF3B1 identified by Next-Generation sequencing have pathogenic as well as prognostic significance, the importance of these mutations has not been validated in prospective trials and their use is therefore not recommended in routine practice.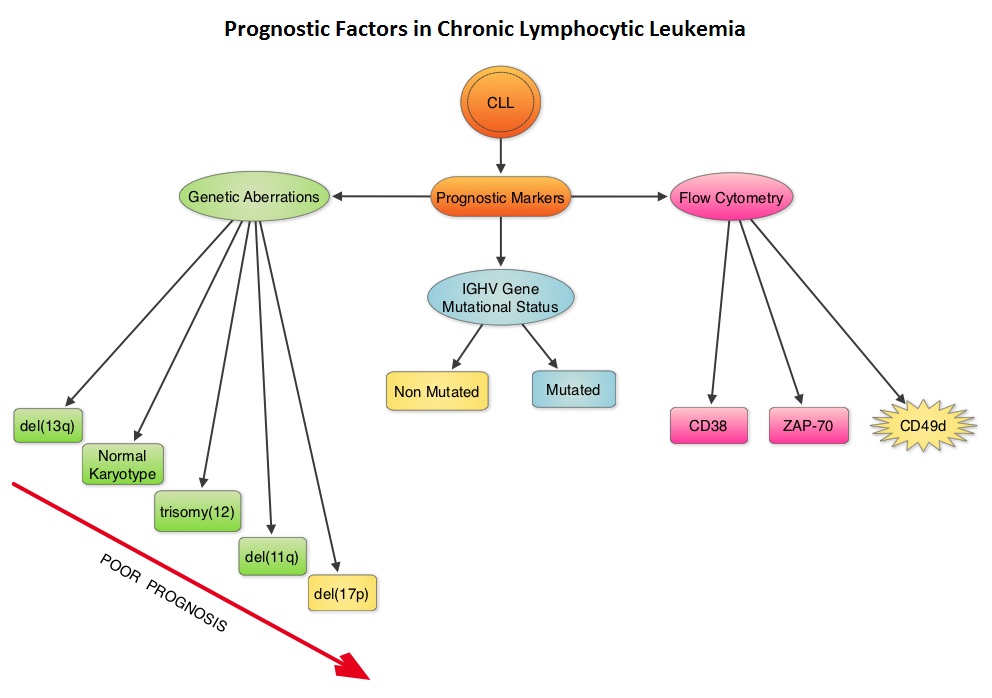
IGHV Mutational status
Retrospective studies have suggested that patients with CLL whose leukemic cells have clonotypically rearranged immunoglobulin genes in germline configuration (Unmutated IGHV gene) demonstrated more aggressive disease and shorter survival time compared to those patients with somatic hypermutations in their IGHV genes (Mutated IGHV gene). The presence of mutated IGHV genes, when combined with additional prognostic factors such as favorable cytogenetics or attainment of a Minimal Residual Disease (MRD) negative state after therapy, characterizes a subgroup of CLL patients with excellent outcome following chemoimmunotherapy with Fludarabine, Cyclophosphamide, and Rituximab. Assessment of IGHV stereotypes however is presently not a part of the routine prognostic work up in CLL.
Serum Biomarkers
Serum markers such as levels of soluble CD23, Thymidine Kinase, and Beta 2-microglobulin are poor prognostic factors and have been shown in several studies to be associated with inferior Overall Survival or Progression Free Survival. Of these, Beta 2-microglobulin has retained independent prognostic value in several multiparameter scores. Assays for these markers should be standardized, and used in prospective clinical trials to validate their relative value in the management of patients with CLL.
Clinical Staging
The two widely accepted staging systems for use in both patient care and clinical trials, the Rai and Binet staging systems rely solely on a physical examination and standard laboratory tests and do not require imaging studies. They are simple and inexpensive and can be readily and consistently applied by physicians worldwide.
Response Definition after Treatment of CLL patients
Assessment of response should include a careful physical examination and evaluation of the blood and bone marrow. For continued therapies or treatment strategies that contain a maintenance phase, the assessment of response should be performed at least 2 months after patients achieve their maximum response or at a time point that is predefined in the protocol and it is not necessary to interrupt therapy for response assessment. The updated guidelines also recommended monitoring for lymphadenopathy, splenomegaly, and hepatomegaly to define relapsed disease and treatment failure, and suggested that the use of imaging in CLL does not typically add much information to the detection of progression or relapse.
MRD Assessment
The desired outcome is complete eradication of the leukemic cells.It was recommended that MRD assessment via multicolor Flow Cytometry, Polymerase Chain Reaction, or Next-Generation sequencing after therapy, be evaluated in the blood and bone marrow, and in clinical trials should be reported with the intent-to-treat population as the denominator and not as a proportion of the responders.
Antiviral Prophylaxis
Patients treated with agents such as Alemtuzumab and Idelalisib (alone or in combination) should be monitored for symptoms or laboratory evidence of opportunistic infections such as Pneumocystis jiroveci or Herpes viridae (Herpes Simplex virus, Varicella-Zoster virus, Cytomegalo virus, Epstein-Barr virus). HBV serological status should be evaluated before treatment with anti-CD20 antibodies as patients may experience reactivation of HBV infections. Appropriate antiviral prophylaxis should be initiated in patients with a history of HBV infection. Infections with JC virus should be ruled out in situations of unclear neurological symptoms, as Progressive Multifocal Leukoencephalopathy has been reported in a few CLL patients treated with anti-CD20 antibodies.
iwCLL guidelines for diagnosis, indications for treatment, response assessment, and supportive management of CLL. Hallek M, Cheson BD, Catovsky D, et al.Blood 2018 131:2745-2760

