SUMMARY: Lung cancer is the second most common cancer in both men and women and accounts for about 13% of all new cancers and 27% of all cancer deaths. It is the leading cause of cancer death among both men and women. The American Cancer Society’s estimates that over 224,000 new cases of lung cancer will be diagnosed in the United States in 2014 and over 159,000 will die of the disease. With changes in the cigarette composition and decline in tobacco consumption over the past several decades, adenocarcinoma now is the most frequent histologic subtype of lung cancer. In the mid 1990’s, following a meta-analysis of 52 randomized clinical trials, platinum based doublet chemotherapy became the accepted standard, for patients with Stage IV Non Small Cell Lung Cancer (NSCLC), after this combination demonstrated a 27% reduction in the risk of death compared to supportive care. Since then, significant advances have been made and it is now established that Platinum/Pemetrexed (ALIMTA®) combination results in superior survival in those with non squamous histology tumors whereas Platinum/ Gemcitabine (GEMZAR®) combination is superior in patients with squamous cell histology. In 2004, the discovery of Epidermal Growth Factor Receptor (EGFR) mutations in some advanced NSCLC cases with adenocarcinoma histology and the favorable responses with EGFR Tyrosine Kinase Inhibitors (TKIs) such as TARCEVA® (Erlotinib) and IRESSA® (Gefitinib), changed the treatment paradigm in favor of targeted therapy for this patient subset. Subsequently, the discovery of rearrangements of the Anaplastic Lymphoma Kinase (ALK) gene in some patients with advanced NSCLC and adenocarcinoma histology, led to the development of agents such as XALKORI® (Crizotinib) and ZYKADIA® (Ceritinib), with promising results. It has become clear that appropriate, molecularly targeted therapy for tumors with a molecular abnormality, results in the best outcomes. This new paradigm lead to the development of evidence based recommendations by a panel of experts in thoracic oncology taking the “driver oncogenes and driver mutations” into consideration. According to the US Lung Cancer Mutation Consortium (LCMC), two thirds of patients with advanced adenocarcinoma of the lung, have a molecular driver abnormality. The most common oncogenic drivers in patients with advanced adenocarcinoma of the lung are, KRAS in 25%, EGFR in 21% and ALK in 8% as well as other mutations in BRAF, HER2, AKT1 and fusions involving RET and ROS oncogenes. These mutations are mutually exclusive and the presence of two simultaneous mutations, are rare. The following guidelines have been put together, to better manage patients with advanced adenocarcinoma of the lung. 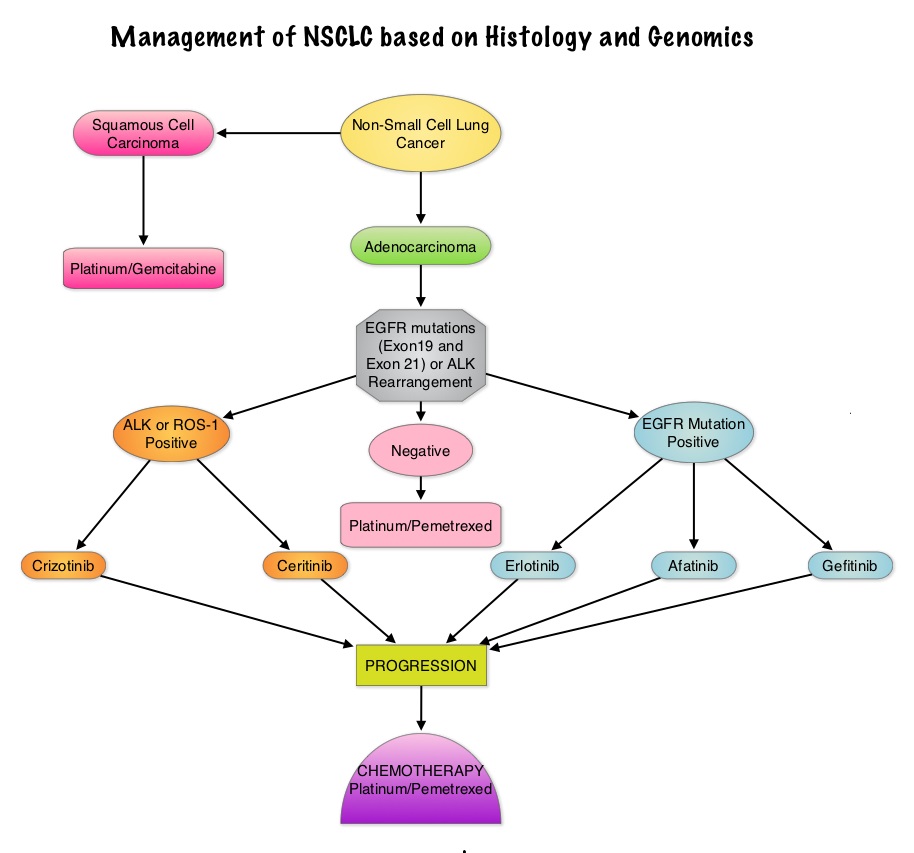
EGFR Mutations and EGFR TKIs
• All patients with advanced NSCLC with the exclusion of pure squamous cell carcinoma should be tested for EGFR mutations before first line treatment decision is made. Because EGFR mutations and ALK rearrangement are mutually exclusive, routine testing of the other is not required for those patients with one known genomic abnormality.
• Patients should be tested for the most common sensitizing mutations such as deletions in exon 19 and L858R point mutations in exon 21, as these patients clearly benefit from first line EGFR TKIs. EGFR expression by IHC (ImmunoHistoChemical) staining, EGFR gene copy by FISH (Fluorescence In Situ Hybridization) and blood based proteonomic testing by VERISTRAT® is currently not recommended for the selection of first line EGFR TKIs.
• Any one of the three available agents, TARCEVA® (Erlotinib), GILOTRIF® (Afatinib) or IRESSA® (Gefitinib) are recommended for first line treatment, as per the regulatory label, because of the absence of data on direct comparisons.
• Platinum based chemotherapy should be considered after EGFR TKI failure in eligible patients and there is no evidence to recommend a preferred chemotherapy regimen in EGFR mutation positive patients.
• If EGFR mutation positive results become available after commencement of first line chemotherapy, early interruption of chemotherapy is discouraged. However maintenance therapy with EGFR TKI should be considered after completion of first line chemotherapy.
• In patients with unknown EGFR mutational status, first line platinum based chemotherapy is the standard of care.
• Continuing EGFR TKI beyond disease progression after its use as first line treatment is not recommended.
ALK Rearrangements and Treatment Selection
• Currently, ALK status is determined by FISH technique although ALK testing by IHC analysis is gaining momentum.
• All patients with advanced NSCLC with the exclusion of pure squamous cell carcinoma should be tested for ALK rearrangement before decision about first line treatment is made. Because EGFR mutations and ALK rearrangements are mutually exclusive, routine testing of the other is not required for those patients with one known genomic abnormality.
• In the US, XALKORI® (Crizotinib) is approved for use in any line of treatment.
• Chemotherapy is allowed in any line of treatment although it is preferable to use it following treatment failure with XALKORI®.
• ZYKADIA® (Ceritinib) is approved in the US for treatment of patients with disease progression on or who are intolerant to XALKORI® (Crizotinib).
• If ALK positive results become available after commencement of first line chemotherapy, early interruption of chemotherapy is discouraged. In these patients, XALKORI® should be used as second line treatment following disease progression on chemotherapy. Maintenance therapy with XALKORI® is not recommended.
• In clinical practice, a repeat biopsy is not recommended at disease progression after treatment with EGFR TKIs or ALK inhibitors.
Gridelli C, de Marinis F, Cappuzzo F, et al. Clinical Lung Cancer 2014;15:173-181

