SUMMARY: Breast cancer is the most common cancer among women in the US and about 1 in 8 women (12%) will develop invasive breast cancer during their lifetime. It is estimated that 252,710 new cases of invasive breast cancer and 63,410 new cases of non-invasive breast cancer will be diagnosed in women in 2017 and 40,610 women are expected to die from the disease. Triple Negative Breast Cancer (TNBC) is a heterogeneous, molecularly diverse group of breast cancers and are ER (Estrogen Receptor), PR (Progesterone Receptor) and HER2 (Human Epidermal Growth Factor Receptor 2) negative. TNBC accounts for 15% to 20% of invasive breast cancers, with a higher incidence noted in young patients. It is usually aggressive, and tumors tend to be high grade and patients with TNBC are at a higher risk of both local and distant recurrence. Those with metastatic disease have one of the worst prognoses of all cancers with a median Overall Survival of 13 months. The majority of patients with TNBC who develop metastatic disease do so within the first 3 years after diagnosis, whereas those without recurrence during this period of time have survival rates similar to those with ER-positive breast cancers. The lack of known recurrent oncogenic drivers in patients with metastatic TNBC, presents a major therapeutic challenge. Nonetheless, patients with TNBC often receive chemotherapy in the neoadjuvant, adjuvant or metastatic settings and approximately 30-40% of patients achieve a pathological Complete Response (pCR) in the neoadjuvant setting. Those who do not achieve a pathological Complete Response tend to have a poor prognosis. It therefore appears that there are subsets of patients with TNBC who may be inherently insensitive to cytotoxic chemotherapy. Three treatment approaches appear to be promising and they include immune therapies, PARP inhibition and inhibition of PI3K pathway.
Using gene expression profiling, TNBC can be classified into 4 distinct molecular subtypes- two Basal-Like (BL1, BL2), Mesenchymal type (M) and Luminal Androgen Receptor type (LAR). BL1 molecular subtype of TNBCs are characterized by high levels of expression of genes involved in the cell cycle and DNA-damage repair pathways and accounts for up to 18% of TNBCs. These tumors are more sensitive to therapies targeting the DNA-repair pathways such as platinum based chemotherapy and Poly-ADP Ribose Polymerase (PARP) inhibition. BL2 molecular subtype of TNBCs represent 13% of TNBCs and in contrast are characterized by upregulation of growth factor signaling pathways, including the Epidermal Growth Factor (EGF), MET pathways, as well as genes involved in glycolysis and gluconeogenesis. These tumors may better respond to small molecule inhibitors of growth factor pathways. An alternate classification of Basal-Like subtype includes Basal-Like Immune Suppressed (BLIS) which is associated with downregulation of B cell, T cell, and Natural Killer cell immune-regulating pathways, and has the worse prognosis and Basal-Like Immune Activated (BLIA) subtype, which has the best prognosis due to upregulated immune-associated pathways. Mesenchymal TNBCs constitute approximately 10-30% of TNBC tumors and are associated with aberrations in the PI3K/AKT/ mTOR pathway as well as increased angiogenesis and may benefit from agents targeting these pathways. Metaplastic breast cancer belongs to this TNBC group. The Luminal Androgen Receptor (LAR) subtype accounts for approximately 11% of TNBCs. These tumors have a high expression of Androgen Receptor by IHC (Immuno HistoChemistry) and benefit from Androgen Receptor blockade and do not respond to cytotoxic chemotherapy.
Ipatasertib is a highly selective oral ATP-competitive, small-molecule, AKT inhibitor and sensitivity to Ipatasertib has been associated with high levels of phosphorylated AKT, PTEN protein loss or genetic mutations in PTEN, and PIK3CA mutations. KRAS and BRAF mutations are typically associated with resistance to Ipatasertib. It is estimated that approximately 50% of TNBCs have deficient expression of the tumor suppressor PTEN, which is associated with a higher degree of AKT pathway activation Preclinical studies showed synergy between Ipatasertib and Taxanes. Because of the high prevalence of PI3K/AKT pathway activation in TNBCs, the authors in this study evaluated the benefit of a combination of Ipatasertib and Paclitaxel as first line therapy, for TNBC.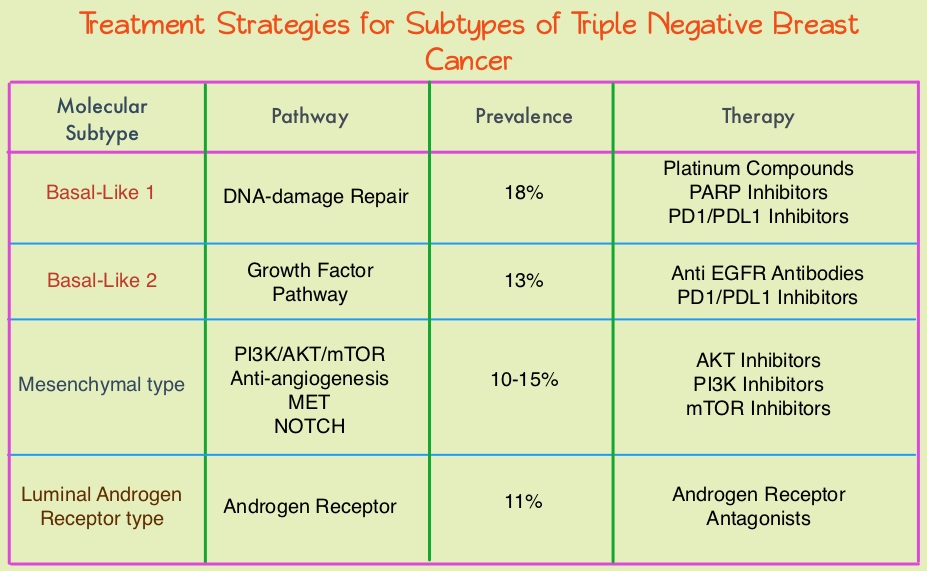
The LOTUS trial is a randomized, placebo controlled, double blind, phase II study in which 124 treatment naïve patients with inoperable, locally advanced or metastatic Triple Negative Breast Cancer were randomly assigned (1:1) to receive Paclitaxel 80 mg/m2 IV Days 1, 8, 15 of a 28 day cycle in combination with either Ipatasertib 400 mg PO daily (N=62) or placebo (N=62), administered on days 1-21 of each 28 day cycle. Treatment was continued until disease progression or unacceptable toxicity. Patients in this study were stratified based on expression of the PTEN tumor suppressor gene and alteration of PIK3CA/AKT1/PTEN in their tumors. The co-primary endpoints were Progression Free Survival (PFS) in the intent-to-treat population and Progression Free Survival in the PTEN-low population. Secondary endpoints included Objective Response Rate and Duration of Response. The median follow up was 10.3 months.
It was noted that the median PFS in the intent-to-treat population was 6.2 months with Ipatasertib versus 4.9 months with placebo (HR=0.60; P=0.037). In the 48 patients with low PTEN expression tumors, the median PFS however was 6.2 months with Ipatasertib and 3.7 months with placebo (HR=0.59; P=0.18), and this was not statistically significant. The PFS benefit was more pronounced in the patient group with PIK3CA/AKT1/PTEN-altered tumors, with a median PFS of 9.0 months in the Ipatasertib group versus 4.9 months in the placebo group (HR=0.44; P=0.041). The most common grade 3/4 adverse events in the Ipatasertib group were diarrhea and neutropenia.
The authors concluded that Ipatasertib prolonged Progression Free Survival compared to placebo and is the first study supporting AKT-targeted therapy for Triple Negative Breast Cancer, supporting the use of gene expression profiling in this heterogeneous malignancy. Ipatasertib plus paclitaxel versus placebo plus paclitaxel as first-line therapy for metastatic triple-negative breast cancer (LOTUS): a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Kim S, Dent R, Im S, et al on behalf of the LOTUS investigators. The Lancet Oncology 2017;18:1360-1372

