SUMMARY: Non-Hodgkin Lymphoma (NHL) is one of the most common cancers in the United States and the American Cancer Society estimates that in 2014, about 70,800 people will be diagnosed with NHL in the US and close to 19,000 people will die of the disease. RITUXAN® (Rituximab) is a first generation type I, chimeric anti-CD20 targeted monoclonal antibody that destroys malignant human B cells primarily by complement-dependent cytotoxicity (CDC) and Antibody-Dependent Cell-Mediated Cytotoxicity (ADCC).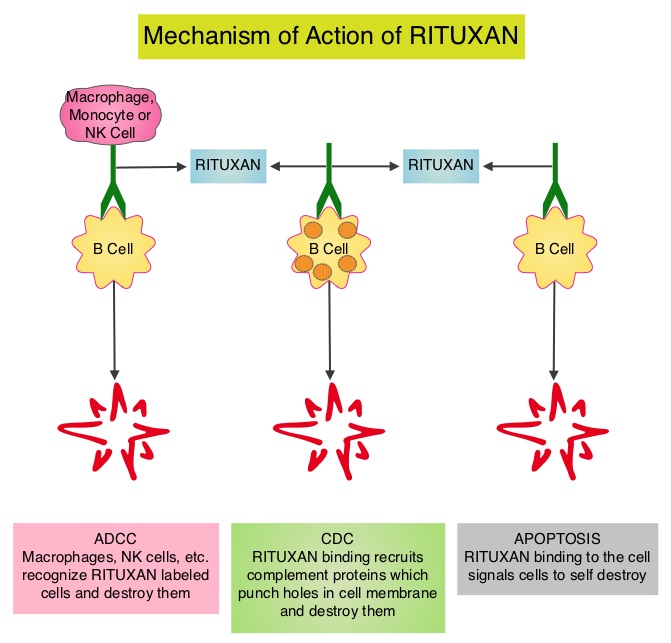 Since its approval in 1997, immunochemotherapy regimens incorporating RITUXAN® has had a major impact in treatment outcomes for patients with Follicular Lymphomas both in first line as well as relapsed settings. Two years of RITUXAN® maintenance therapy after induction immunochemotherapy as first-line treatment for high tumor burden Follicular Lymphoma, significantly improved Progression Free Survival, as was shown in the PRIMA study. Similarly, maintenance RITUXAN® has been shown to improve Progression Free Survival when compared with observation, in patients with low tumor burden Follicular Lymphoma. Whether maintenance RITUXAN® provides superior long term disease control compared with retreatment with RITUXAN® when disease progression is noted, has remained unclear. RESORT [Rituximab Extended Schedule or Re-Treatment Trial] is a randomized trial designed to determine whether maintenance treatment with RITUXAN® provided superior disease control compared with retreatment with RITUXAN® at disease progression, in patients with previously untreated low tumor burden Follicular Lymphoma. Low tumor burden was defined as no mass more than 7 cm, fewer than three masses more than 3 cm, no B symptoms, spleen size less than 16 cm by CT scan, no evidence of organ compromise, circulating lymphocytes less 5,000/μL, and no evidence of cytopenias defined as platelets less than 100,000/μL, hemoglobin less than 10 g/dL, or absolute neutrophil count less than 1,500/μL. Of the 408 patients with Follicular Lymphoma included in this study, 289 patients responded to induction treatment with 4 weekly doses of RITUXAN® given at 375mg/m2. These patients were then randomly assigned to maintenance RITUXAN® (N= 146) or retreatment with RITUXAN® (N=143) at each disease progression, until treatment failure. Maintenance RITUXAN® treatment consisted of a single dose of RITUXAN® given every 3 months until treatment failure. The primary end point of this study was time to treatment failure. Secondary end points included time to first cytotoxic therapy, toxicity, and health-related quality of life (HRQOL). With a median follow-up of 4.5 years, there was no difference in the median time to treatment failure amongst the maintenance RITUXAN® and retreatment RITUXAN® groups (4.3 years vs 3.9 years, P=0.54). The median number of RITUXAN® doses was 18 for those receiving maintenance RITUXAN® compared to 4 for those receiving retreatment RITUXAN®. Grade 3 or 4 toxicities were uncommon in both treatment groups and there was no difference in health-related quality of life. The authors concluded that in low tumor burden Follicular Lymphoma, a retreatment strategy at disease progression utilizes fewer doses of RITUXAN® with outcomes equivalent to that achieved with maintenance RITUXAN®. Kahl BS, Hong F, Williams ME, et al. J Clin Oncol 2014;32:3096-3102
Since its approval in 1997, immunochemotherapy regimens incorporating RITUXAN® has had a major impact in treatment outcomes for patients with Follicular Lymphomas both in first line as well as relapsed settings. Two years of RITUXAN® maintenance therapy after induction immunochemotherapy as first-line treatment for high tumor burden Follicular Lymphoma, significantly improved Progression Free Survival, as was shown in the PRIMA study. Similarly, maintenance RITUXAN® has been shown to improve Progression Free Survival when compared with observation, in patients with low tumor burden Follicular Lymphoma. Whether maintenance RITUXAN® provides superior long term disease control compared with retreatment with RITUXAN® when disease progression is noted, has remained unclear. RESORT [Rituximab Extended Schedule or Re-Treatment Trial] is a randomized trial designed to determine whether maintenance treatment with RITUXAN® provided superior disease control compared with retreatment with RITUXAN® at disease progression, in patients with previously untreated low tumor burden Follicular Lymphoma. Low tumor burden was defined as no mass more than 7 cm, fewer than three masses more than 3 cm, no B symptoms, spleen size less than 16 cm by CT scan, no evidence of organ compromise, circulating lymphocytes less 5,000/μL, and no evidence of cytopenias defined as platelets less than 100,000/μL, hemoglobin less than 10 g/dL, or absolute neutrophil count less than 1,500/μL. Of the 408 patients with Follicular Lymphoma included in this study, 289 patients responded to induction treatment with 4 weekly doses of RITUXAN® given at 375mg/m2. These patients were then randomly assigned to maintenance RITUXAN® (N= 146) or retreatment with RITUXAN® (N=143) at each disease progression, until treatment failure. Maintenance RITUXAN® treatment consisted of a single dose of RITUXAN® given every 3 months until treatment failure. The primary end point of this study was time to treatment failure. Secondary end points included time to first cytotoxic therapy, toxicity, and health-related quality of life (HRQOL). With a median follow-up of 4.5 years, there was no difference in the median time to treatment failure amongst the maintenance RITUXAN® and retreatment RITUXAN® groups (4.3 years vs 3.9 years, P=0.54). The median number of RITUXAN® doses was 18 for those receiving maintenance RITUXAN® compared to 4 for those receiving retreatment RITUXAN®. Grade 3 or 4 toxicities were uncommon in both treatment groups and there was no difference in health-related quality of life. The authors concluded that in low tumor burden Follicular Lymphoma, a retreatment strategy at disease progression utilizes fewer doses of RITUXAN® with outcomes equivalent to that achieved with maintenance RITUXAN®. Kahl BS, Hong F, Williams ME, et al. J Clin Oncol 2014;32:3096-3102

