SUMMARY: The American Cancer Society estimates that for 2019, about 56,770 people will be diagnosed with pancreatic cancer and about 45,750 people will die of the disease. Pancreatic cancer is the fourth most common cause of cancer-related deaths in the United States and Western Europe. Unfortunately, unlike other malignancies, very little progress has been made and outcome for patients with advanced pancreatic cancer has been dismal, with a 5-year survival rate for metastatic pancreatic cancer of approximately 2%. Pancreatic cancer has surpassed breast cancer as the third leading cause of cancer death in the United States and is on track to surpass colorectal cancer, to move to the second leading cause of cancer related deaths in the United States around 2020.
BRCA1 and BRCA2 are tumor suppressor genes located on chromosome 17 and chromosome 13 respectively. They control cell growth by repairing DNA damage and thus prevent tumor development. Mutations in these genes predispose an individual to develop malignant tumors. It is well established that the presence of BRCA1 and BRCA2 mutations can significantly increase the lifetime risk for developing breast and ovarian cancer, as high as 85% and 40% respectively. BRCA1/2 mutations have been detected in 4-7% of patients with pancreatic cancer, with a 2-6 fold increase in risk, associated with these mutations. These patients tend to be younger. Among pancreatic cancer patients with Ashkenazi Jewish ancestry, the prevalence of BRCA1/2 mutations is 6-19%, with mutations more common for BRCA2. NCCN guideline recommends that germline testing should be considered for all patients with pancreatic cancer and is especially recommended for those with a personal history of cancer, family history or clinical suspicion of a family history of pancreatic cancer. Approximately 10% of pancreatic cancer cases have a familial component. When hereditary cancer syndrome is suspected in patients with pancreatic cancer, genetic counseling should be considered.
BRCA mutations can either be inherited (Germline) and present in all individual cells or can be acquired and occur exclusively in the tumor cells (Somatic). The BRCA gene plays an important role in DNA repair via Homologous Recombination (HR). Mutation of BRCA gene results in loss of BRCA function and likely deregulates Homologous Recombination (HR) pathway. Majority of patients with Germline BRCA mutations (gBRCA) have HR Deficiency (HRD) resulting in inability to repair double strand breaks. HRD can also occur due to other mechanisms, such as germline mutations, somatic mutations and epigenetic modifications of other genes involved in the HR pathway. Patients with HRD exhibit specific clinical behaviors, and improved responses to treatments, such as platinum-based chemotherapy and PARP Inhibitors. 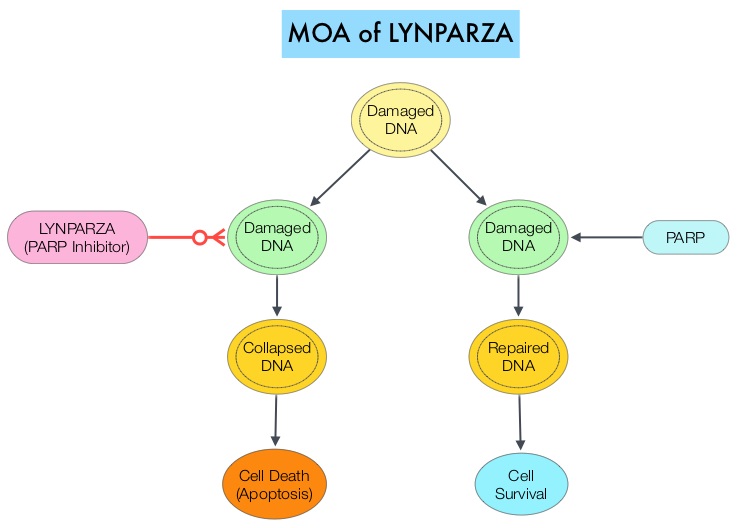
The PARP (Poly ADP Ribose Polymerase) family of enzymes include PARP1 and PARP2, which repair damaged DNA. LYNPARZA® is a first-in-class PARP enzyme inhibitor that causes cell death in tumors that already have a DNA repair defect, such as those with BRCA1 and BRCA2 mutations, through the concept of synthetic lethality. Malignancies such as epithelial ovarian cancers with Homologous Recombination Deficiency have demonstrated sensitivity to PARP inhibitors. Recent studies have confirmed that PARP inhibitors are effective not only in ovarian cancers displaying germline or somatic BRCA mutations but also in cancers with HRD caused by other underlying etiologies. LYNPARZA® in a Phase II trial, demonstrated antitumor activity in heavily pretreated metastatic pancreatic cancer patients with a germline BRCA mutation. Maintenance treatment with LYNPARZA® in BRCA mutated ovarian cancer patients resulted in significant improvement in Progression Free Survival.
The POLO (Pancreas Cancer Olaparib Ongoing) trial was conducted to evaluate the efficacy of maintenance therapy with LYNPARZA® in metastatic pancreatic adenocarcinoma patients with a germline BRCA mutation whose disease had not progressed during first-line platinum-based chemotherapy. In this international, multicenter, randomized, double-blind, placebo-controlled Phase III study, 154 patients with BRCA mutant disease were randomly assigned in a 3:2 ratio, to receive maintenance LYNPARZA® tablets 300 mg twice daily (N=92) or matching placebo (N=62). The median patient age was 57 years. Eligible patients should have received at least 16 weeks of continuous first-line platinum-based chemotherapy for metastatic pancreatic cancer and maintenance treatment was initiated 4-8 weeks after the last dose of first-line chemotherapy had been administered. Maintenance intervention was continued until disease progression. Crossover to LYNPARZA® was not permitted during this trial. The Primary end point was Progression Free Survival and Secondary end points included Objective Response Rate (ORR) and Quality of Life.
The median PFS was significantly longer in the LYNPARZA® group compared to the placebo group (7.4 months versus 3.8 months; HR for disease progression or death=0.53; P=0.004). This suggested a 47% reduction in the risk of disease progression or death. At 2 years, 22% of the patients in the LYNPARZA® group did not have disease progression compared with 9.6% of patients in the placebo group. The interim analysis of Overall Survival showed no significant difference, with a median 18.9 months for the LYNPARZA® group and 18.1 months for the placebo group (HR=0.91; P=0.68). Health-related Quality of Life scores were also not significantly different. Grade 3 or higher adverse events were 40% in the LYNPARZA® group and 23% in the placebo group and 5% and 2% of the patients, respectively, discontinued therapy because of an adverse event.
It was concluded that among metastatic pancreatic cancer patients with germline BRCA mutation and whose cancer has not progressed during platinum-based chemotherapy, Progression Free Survival was significantly longer with maintenance LYNPARZA® than with placebo. This study allows identifying patients with metastatic pancreatic cancer who will likely benefit from PARP inhibition. Maintenance Olaparib for Germline BRCA-Mutated Metastatic Pancreatic Cancer. Golan T, Hammel P, Reni M, et al. N Engl J Med 2019; 381:317-327

