SUMMARY: It is estimated that approximately 5000 new cases of GastroIntestinal Stromal Tumors (GISTs) will be diagnosed in the United States in 2015. Even though most of the GIST cases are sporadic, hereditary GIST has been well documented in individuals with von Recklinghausen’s neurofibromatosis as well as a familial syndrome in children and as a rare constellation of gastric GIST, pulmonary chondroma and extra adrenal paraganglioma (Carney’s Triad). Presently GLEEVEC® (Imatinib) is approved by the FDA for the adjuvant treatment of adult patients following resection of Kit (CD117) positive GIST and SUTENT® (Sunitinib) is approved in this setting if patients are intolerant to GLEEVEC®. GISTs are mesenchymal neoplasms and originate from Interstitial Cells of Cajal (ICC) or their precursors. These cells normally regulate GI motility ie. peristaltic wave and are considered pacemaker cells of the gut. They are located throughout the GI tract and transmit signals along the bowel.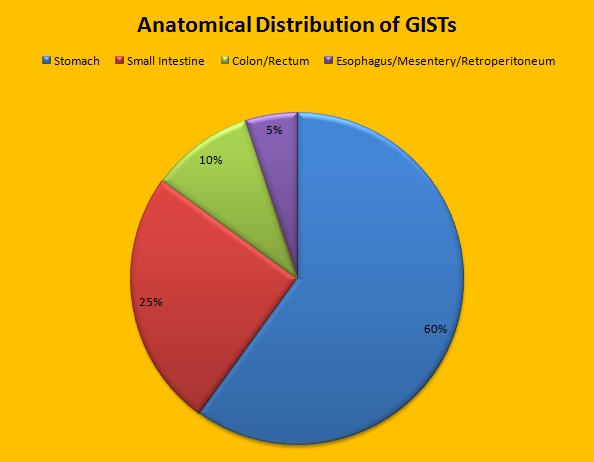 Approximately 60% of the primary GISTs originate in the stomach, 25% in the small intestine, 10% in the colon and rectum and the rest at other sites such as the esophagus, mesentery and retroperitoneum. GISTs were misclassified as leiomyomas or leiomyosarcomas until 1998, when the discovery of mutations in the c-KIT oncogene, lead to a better understanding of these tumors. C-KIT oncogene encodes the transmembrane KIT receptor tyrosine kinase. Approximately 85% of the GISTs have activating (gain of function) KIT mutations and are positive for the CD117 antigen, an epitope of KIT receptor tyrosine kinase. Positive CD117 by ImmunoHistoChemistry (IHC) is however not specific for GIST, as weak reactivity occurs with other mesenchymal tumors. IHC staining for protein kinase C theta and DOG1 are helpful in distinguishing GIST from other mesenchymal tumors, particularly those that are KIT-negative. DOG1 (Discovered On GIST 1) is a protein of unknown function that is expressed strongly in GISTs and is rarely expressed in other mesenchymal neoplasms. KIT mutations in GISTs occur in Exon 9 (10%), Exon 11 (67%), Exon 13 (1%) and Exon 17 (1%). About 5% of the GISTs have activating mutation in the Platelet-Derived Growth Factor Receptor alpha (PDGFRA) gene which encodes for another tyrosine kinase receptor. Approximately 10% to 20% of GISTs have no KIT or PDGFRA mutations and are referred as wild-type GISTs. Mutational status is important as they may predict response to GLEEVEC® and also have prognostic significance. Patients with KIT exon 11 mutations are most sensitive and have a much higher response to GLEEVEC® whereas those with KIT exon 9 mutation or wild-type c-KIT may require a higher dose of GLEEVEC® (800 mg daily dose). KIT exon 11 mutations affecting the codons 557 and 558 is an independent adverse prognostic factor and associated with higher incidence of metastases whereas GISTs with KIT exon 9 mutations usually arise in the small bowel and are associated with frequent recurrences. GISTs with PDGFRA mutations in general have low mitotic count and low malignant potential and those with PDGFRA exon 18 mutation have favorable survival outcomes and located in the stomach. They are however resistant to GLEEVEC®. Although mutated KIT and PDGFRA have been identified as important driver mutations for GIST oncogenesis, the clinical significance of their single mutations has been unclear. To address this, the authors in this study identified 3067 patients with GIST from databases who had macroscopically complete tumor excision and had no detectable metastases at the time of diagnosis. Information on mutation analysis was available on 1505 tumors. The researchers then analyzed associations between KIT and PDGFRA mutations and Recurrence Free Survival (RFS) in this patient population treated with surgery alone.
Approximately 60% of the primary GISTs originate in the stomach, 25% in the small intestine, 10% in the colon and rectum and the rest at other sites such as the esophagus, mesentery and retroperitoneum. GISTs were misclassified as leiomyomas or leiomyosarcomas until 1998, when the discovery of mutations in the c-KIT oncogene, lead to a better understanding of these tumors. C-KIT oncogene encodes the transmembrane KIT receptor tyrosine kinase. Approximately 85% of the GISTs have activating (gain of function) KIT mutations and are positive for the CD117 antigen, an epitope of KIT receptor tyrosine kinase. Positive CD117 by ImmunoHistoChemistry (IHC) is however not specific for GIST, as weak reactivity occurs with other mesenchymal tumors. IHC staining for protein kinase C theta and DOG1 are helpful in distinguishing GIST from other mesenchymal tumors, particularly those that are KIT-negative. DOG1 (Discovered On GIST 1) is a protein of unknown function that is expressed strongly in GISTs and is rarely expressed in other mesenchymal neoplasms. KIT mutations in GISTs occur in Exon 9 (10%), Exon 11 (67%), Exon 13 (1%) and Exon 17 (1%). About 5% of the GISTs have activating mutation in the Platelet-Derived Growth Factor Receptor alpha (PDGFRA) gene which encodes for another tyrosine kinase receptor. Approximately 10% to 20% of GISTs have no KIT or PDGFRA mutations and are referred as wild-type GISTs. Mutational status is important as they may predict response to GLEEVEC® and also have prognostic significance. Patients with KIT exon 11 mutations are most sensitive and have a much higher response to GLEEVEC® whereas those with KIT exon 9 mutation or wild-type c-KIT may require a higher dose of GLEEVEC® (800 mg daily dose). KIT exon 11 mutations affecting the codons 557 and 558 is an independent adverse prognostic factor and associated with higher incidence of metastases whereas GISTs with KIT exon 9 mutations usually arise in the small bowel and are associated with frequent recurrences. GISTs with PDGFRA mutations in general have low mitotic count and low malignant potential and those with PDGFRA exon 18 mutation have favorable survival outcomes and located in the stomach. They are however resistant to GLEEVEC®. Although mutated KIT and PDGFRA have been identified as important driver mutations for GIST oncogenesis, the clinical significance of their single mutations has been unclear. To address this, the authors in this study identified 3067 patients with GIST from databases who had macroscopically complete tumor excision and had no detectable metastases at the time of diagnosis. Information on mutation analysis was available on 1505 tumors. The researchers then analyzed associations between KIT and PDGFRA mutations and Recurrence Free Survival (RFS) in this patient population treated with surgery alone.
Taking into consideration the tumor size and mitotic rate in addition to mutational analysis data, they made several important observations. Patients with PDGFRA mutations had more favorable RFS than those with KIT mutations (HR=0.34; P =0 .004). They also pointed out that not all KIT Exon 11 mutations are equal and are associated with variable outcomes and clinical features. They concluded that mutation analysis in GISTs may provide clinically useful prognostic information in a patient groups treated with surgery alone, but the standard prognostic factors such as mitotic rate are generally stronger predictors for RFS even in subsets of patients with an identical KIT or PDGFRA mutation. Therefore tumor mutational status should be interpreted in the context of other standard prognostic factors. They did however note that majority of the patients with PDGFRA mutations and those with KIT exon 11 duplication mutation or deletion of one codon have favorable RFS with surgery alone and are usually not candidates for adjuvant therapy. Joensuu H, Rutkowski P, Nishida T, et al. J Clin Oncol 2015; 33:634-642

