SUMMARY: It is estimated that in the US, approximately 76,000 new cases of melanoma will be diagnosed and close to 8000 individuals will die of the disease in 2014. The incidence of melanoma has been on the rise for the past three decades. Unlike other malignancies, the role of chemotherapy for the treatment of melanoma has been limited. Treatment of advanced melanoma with immunotherapy using a cytokine, Interleukin-2 (IL-2) produced by T cells during an immune response, was first explored in the mid 1970’s. Durable responses were noted in a very small percentage of patients but this was associated with significant toxicities. This however opened the doors for the development a novel immunotherapeutic approaches, with a better understanding of the Immune checkpoints.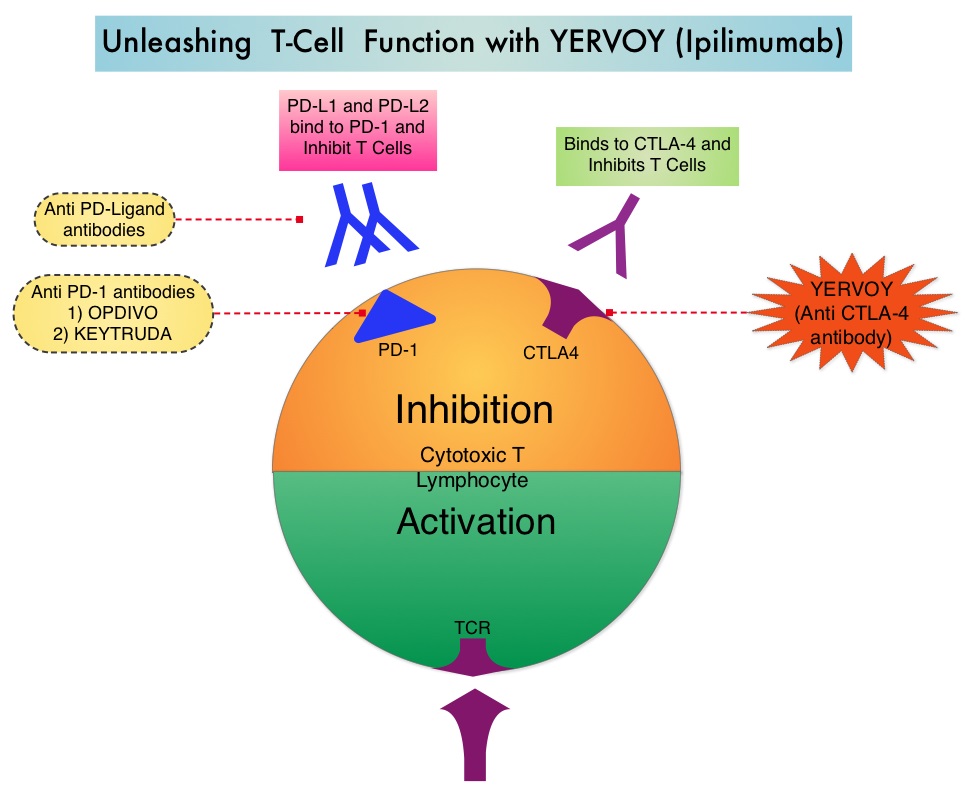 Immune checkpoints are cell surface inhibitory proteins/receptors that are expressed on activated T cells. They harness the immune system and prevent uncontrolled immune reactions. Survival of cancer cells in the human body may be to a significant extent, related to their ability to escape immune surveillance, by inhibiting T lymphocyte activation. The T cells of the immune system therefore play a very important role in modulating the immune system. Under normal circumstances, inhibition of an intense immune response and switching off the T cells of the immune system, is an evolutionary mechanism and is accomplished by Immune checkpoints or gate keepers. With the recognition of Immune checkpoint proteins and their role in suppressing antitumor immunity, antibodies are being developed that target the membrane bound inhibitory Immune checkpoint proteins/receptors such as CTLA-4 (Cytotoxic T-Lymphocyte Antigen 4, also known as CD152), PD-1(Programmed cell Death 1), etc. By doing so, one would expect to unleash the T cells, resulting in T cell proliferation, activation and a therapeutic response. The authors in this randomized study, compared the efficacy of YERVOY® (Ipilimumab) plus Sargramostim with YERVOY® alone, for treatment of metastatic melanoma. The rationale for this study was based on the synergy that was noted between YERVOY® and GM-CSF in preclinical models. The first immune checkpoint protein to be clinically targeted was CTLA-4. YERVOY® is a fully human IgG1monoclonal antibody that blocks Immune checkpoint protein/receptor CTLA- 4 and counteracts immune regulatory cells. YERVOY® has been shown to prolong overall survival in patients with previously treated, unresectable or metastatic melanoma. GM-CSF is a cytokine that enhances the antitumor activity of T and B lymphocytes by activating the antigen presenting dendritic cells and recruiting macrophages. It however can induce negative regulatory immune responses.
Immune checkpoints are cell surface inhibitory proteins/receptors that are expressed on activated T cells. They harness the immune system and prevent uncontrolled immune reactions. Survival of cancer cells in the human body may be to a significant extent, related to their ability to escape immune surveillance, by inhibiting T lymphocyte activation. The T cells of the immune system therefore play a very important role in modulating the immune system. Under normal circumstances, inhibition of an intense immune response and switching off the T cells of the immune system, is an evolutionary mechanism and is accomplished by Immune checkpoints or gate keepers. With the recognition of Immune checkpoint proteins and their role in suppressing antitumor immunity, antibodies are being developed that target the membrane bound inhibitory Immune checkpoint proteins/receptors such as CTLA-4 (Cytotoxic T-Lymphocyte Antigen 4, also known as CD152), PD-1(Programmed cell Death 1), etc. By doing so, one would expect to unleash the T cells, resulting in T cell proliferation, activation and a therapeutic response. The authors in this randomized study, compared the efficacy of YERVOY® (Ipilimumab) plus Sargramostim with YERVOY® alone, for treatment of metastatic melanoma. The rationale for this study was based on the synergy that was noted between YERVOY® and GM-CSF in preclinical models. The first immune checkpoint protein to be clinically targeted was CTLA-4. YERVOY® is a fully human IgG1monoclonal antibody that blocks Immune checkpoint protein/receptor CTLA- 4 and counteracts immune regulatory cells. YERVOY® has been shown to prolong overall survival in patients with previously treated, unresectable or metastatic melanoma. GM-CSF is a cytokine that enhances the antitumor activity of T and B lymphocytes by activating the antigen presenting dendritic cells and recruiting macrophages. It however can induce negative regulatory immune responses. In this phase II randomized clinical trial conducted by the Eastern Cooperative Oncology Group (ECOG), patients with unresectable stage III or IV melanoma (N = 245), who had received at least 1 prior therapy and with no central nervous system metastases were randomized to receive either YERVOY® along with Sargramostim (N=123) or YERVOY® alone (N=122). Patients in the combination group (Group A) received YERVOY®10 mg/kg, IV on day 1 along with Sargramostim 250 μg given subcutaneously, on days 1 thru 14 of a 21day cycle, every 3 weeks for four cycles followed by YERVOY® maintenance every 12 weeks. Patients in Group B received YERVOY® alone. Treatment was continued until disease progression or uncontrolled toxicities. The primary endpoint was comparison of length of Overall Survival (OS). Secondary end points included Progression Free Survival (PFS), response rate, safety, and tolerability. With a median follow up of 13.3 months, the median OS for the combination of YERVOY® plus Sargramostim was 17.5 months vs 12.7 months for YERVOY® alone. The one year survival rate for YERVOY® plus Sargramostim was 68.9% compared to 52.9% for YERVOY® alone (HR=0.64; P=0.01). The median PFS was similar and was 3.1 months in both study groups. The explanation for similar PFS in both treatment groups may be due to both YERVOY® and Sargramostim bringing about inflammatory changes at the tumor sites, which in turn could be misinterpreted as disease progression, on radiological studies. The authors commented that PFS may not be an appropriate endpoint in immunotherapy trials. Grade 3 to 5 adverse events were less in the combination group (44.9%) compared to 58% for single agent YERVOY® (P=0.04). The authors concluded that treatment of unresectable stage III or IV melanoma patients with YERVOY® plus Sargramostim resulted in significantly longer overall survival with lower toxicities, compared to YERVOY® alone. Hodi SF, Lee S, McDermott DF, et al. JAMA 2014;312:1744-1753
In this phase II randomized clinical trial conducted by the Eastern Cooperative Oncology Group (ECOG), patients with unresectable stage III or IV melanoma (N = 245), who had received at least 1 prior therapy and with no central nervous system metastases were randomized to receive either YERVOY® along with Sargramostim (N=123) or YERVOY® alone (N=122). Patients in the combination group (Group A) received YERVOY®10 mg/kg, IV on day 1 along with Sargramostim 250 μg given subcutaneously, on days 1 thru 14 of a 21day cycle, every 3 weeks for four cycles followed by YERVOY® maintenance every 12 weeks. Patients in Group B received YERVOY® alone. Treatment was continued until disease progression or uncontrolled toxicities. The primary endpoint was comparison of length of Overall Survival (OS). Secondary end points included Progression Free Survival (PFS), response rate, safety, and tolerability. With a median follow up of 13.3 months, the median OS for the combination of YERVOY® plus Sargramostim was 17.5 months vs 12.7 months for YERVOY® alone. The one year survival rate for YERVOY® plus Sargramostim was 68.9% compared to 52.9% for YERVOY® alone (HR=0.64; P=0.01). The median PFS was similar and was 3.1 months in both study groups. The explanation for similar PFS in both treatment groups may be due to both YERVOY® and Sargramostim bringing about inflammatory changes at the tumor sites, which in turn could be misinterpreted as disease progression, on radiological studies. The authors commented that PFS may not be an appropriate endpoint in immunotherapy trials. Grade 3 to 5 adverse events were less in the combination group (44.9%) compared to 58% for single agent YERVOY® (P=0.04). The authors concluded that treatment of unresectable stage III or IV melanoma patients with YERVOY® plus Sargramostim resulted in significantly longer overall survival with lower toxicities, compared to YERVOY® alone. Hodi SF, Lee S, McDermott DF, et al. JAMA 2014;312:1744-1753

