SUMMARY: It is estimated that in the US, approximately 76,380 new cases of melanoma will be diagnosed in 2016 and approximately 10,130 patients will die of the disease. The incidence of melanoma has been on the rise for the past three decades. A better understanding of Immune checkpoints has opened the doors for the development of various immunotherapies. Immune checkpoints are cell surface inhibitory proteins/receptors that harness the immune system and prevent uncontrolled immune reactions. Survival of cancer cells in the human body may be related to their ability to escape immune surveillance, by inhibiting T lymphocyte activation. Under normal circumstances, inhibition of an intense immune response and switching off the T cells of the immune system is accomplished by Immune checkpoints or gate keepers. With the recognition of Immune checkpoint proteins and their role in suppressing antitumor immunity, antibodies have been developed that target the membrane bound inhibitory Immune checkpoint proteins/receptors such as CTLA-4 (Cytotoxic T-Lymphocyte Antigen 4, also known as CD152), PD-1(Programmed cell Death 1), etc. By blocking the Immune checkpoint proteins, one would expect to unleash the T cells, resulting in T cell proliferation, activation and a therapeutic response.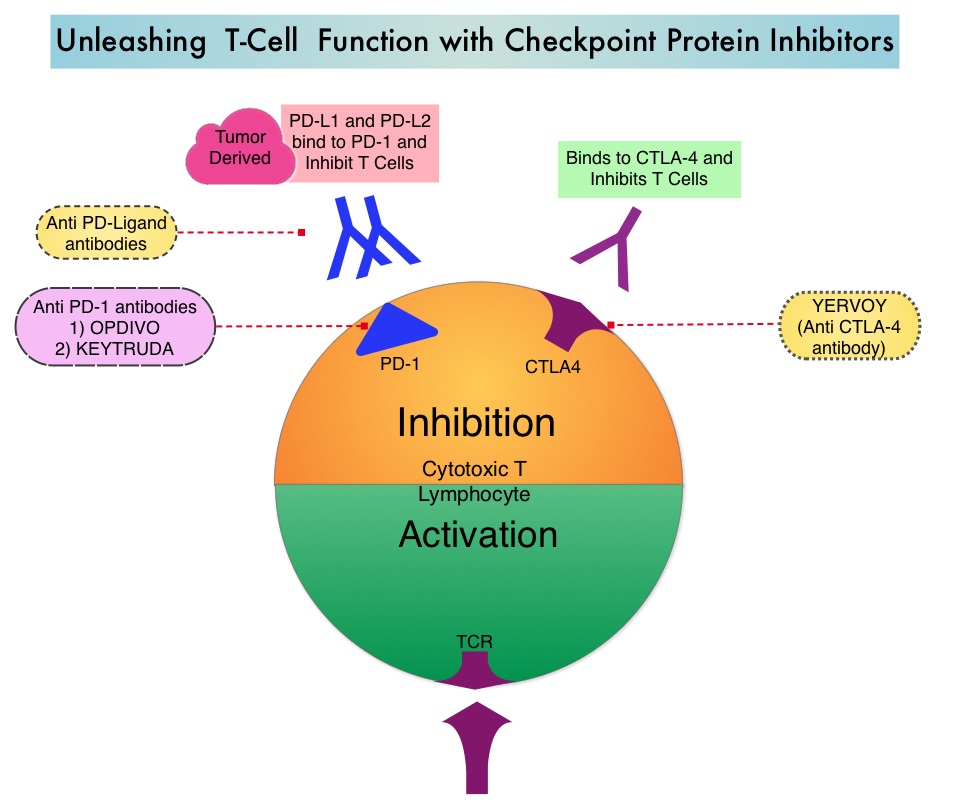
OPDIVO® (Nivolumab) is a fully human, immunoglobulin G4 monoclonal antibody that targets PD-1 receptor. In 2014, Topalian and Colleagues reported their finding of an early phase I trial (J Clin Oncol 2014;32:1020-1030), in which patients with advanced Melanoma (N = 107) who had 1-5 prior systemic therapies received OPDIVO® monotherapy. The median age of patients in this study was 61 years and patients received OPDIVO® every 2 weeks for up to 96 weeks. The median Overall Survival for these patients was 16.8 months and 1 and 2 year survival rates were 62% and 43%, respectively. It was noted that some patients had durable responses that persisted even after treatment was discontinued. This patient group was followed for Overall Survival, Progression Free Survival (PFS), long term safety and response duration, after discontinuing treatment with OPDIVO®. The authors have now reported the results of this extended follow up, with 5 year Overall Survival (OS) data from this study.
The 5 year Overall Survival in all 107 patients was 34% and the median OS was 20.3 months for those who received the approved dose of 3 mg/kg of OPDIVO® and 17.3 months in all 107 patients. As a comparison, according to the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) data, the 5 year OS rate for metastatic Melanoma patients diagnosed during the study period was 16.6%. The OS rates in this study appeared to have plateaued at 48 months. The Progression Free Survival at 30 months following treatment was 25.7% for those who received the approved dose of 3 mg/kg of OPDIVO® and 18.6% for all patients. The most common side effects across the entire cohort included fatigue, rash, diarrhea, pruritus and nausea.
The authors concluded that this analysis represents the first and longest follow up to date, testing an anti–PD1 immunotherapy in any specific disease. Monotherapy with OPDIVO® in heavily pretreated advanced Melanoma patients can result in more than a third of patients (34%) being alive, 5 years after starting treatment. Durable, long-term survival in previously treated patients with advanced melanoma (MEL) who received nivolumab (NIVO) monotherapy in a phase I trial. Hodi FS, Kluger H, Sznol M, et al. 2016 AACR Annual Meeting. Abstract CT001

