SUMMARY: The FDA on October 27, 2015 approved IMLYGIC® (Talimogene laherparepvec or T-VEC), the first FDA-approved oncolytic virus therapy, for the treatment of melanoma lesions in the skin and lymph nodes. The American Cancer Society’s estimates that for 2015, approximately 74,000 new melanomas will be diagnosed in the United States and about 10,000 people are expected to die of the disease. IMLYGIC® is a genetically modified, herpes simplex virus type 1–derived oncolytic immunotherapy designed to induce both local and systemic immune responses. Following injection directly into melanoma lesions, IMLYGIC® selectively replicates within tumors and produces an immunostimulatory protein called Granulocyte-Macrophage Colony Stimulating Factor (GM-CSF). IMLYGIC® causes cell lysis resulting in the release of tumor-derived antigens, which along with the local GM-CSF, recruit and activate antigen-presenting cells, with subsequent induction of tumor-specific T-cell responses. The enhanced systemic antitumor immune response against tumor-derived antigens, eradicates tumor cells elsewhere in the body.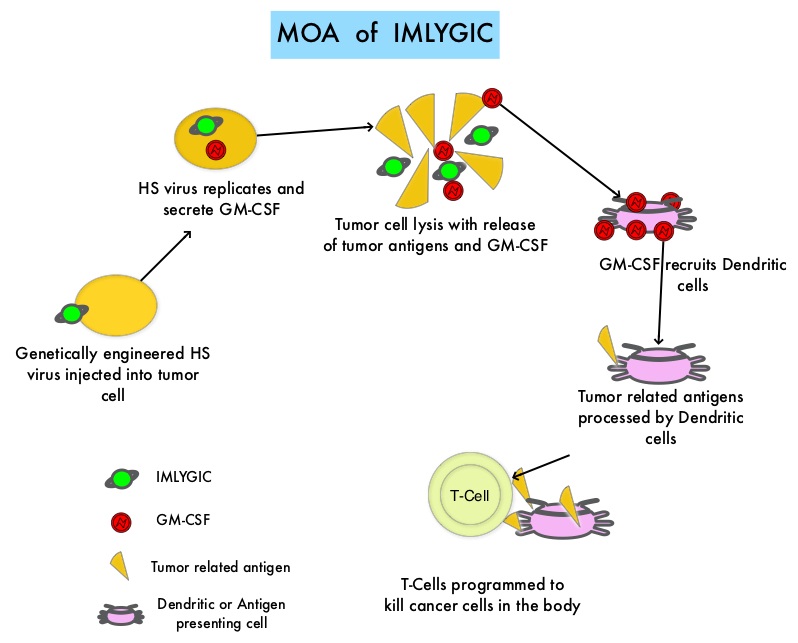
Previously reported single arm phase II study with IMLYGIC® demonstrated an Overall Response Rate (ORR) of 26% in patients with stage IIIC to IV melanoma, with responses noted in both injected and un-injected lesions, including visceral lesions. Oncovex (GM-CSF) Pivotal Trial in Melanoma (OPTiM) is a phase III study in which 436 patients with stage IIIB to IV melanoma, with injectable melanoma lesions that could not be surgically resected, were randomly assigned in a 2:1 ratio to receive intralesional IMLYGIC® (N=295) or subcutaneous GM-CSF (N=141). The enrolled patient’s melanoma lesions in the skin and lymph nodes were treated with IMLYGIC® or a comparator therapy and Injection into visceral lesions was not allowed. Patients in the IMLYGIC® group received a series of injections into the melanoma lesions. Following the initial injection, a second dose was administered three weeks later, followed by additional doses every two weeks for at least six months, unless other treatment was required or until there are were no remaining injectable lesions to treat. Patients in the GM-CSF group received 125 micrograms/m2 subcutaneously once daily for 14 days in 28-day cycles. The median age of patients in the study was 63 years. The primary end point was Durable Response Rate (DRR- Objective Response lasting continuously for 6 months or more). Secondary end points included Overall Survival (OS) and Overall Response Rate.
The DRR was significantly higher among patients receiving IMLYGIC® than among those given GM-CSF (16.3% vs 2.1%; P<0.001). The Overall Response Rate was also higher with IMLYGIC® compared to GM-CSF (26.4% vs 5.7%; P<0.001). Approximately 11% of patients receiving IMLYGIC® experienced a Complete Response compared to less than 1% for those receiving GM-CSF. The median Time to Treatment Failure was 8.2 months with IMLYGIC® and 2.9 months with GM-CSF (HR=0.42) and median Overall Survival was 23.3 months and 18.9 months, respectively (HR=0.79; P=0.051). In an exploratory subset analysis, the benefit with IMLYGIC® was more pronounced among patients with stage IIIB, IIIC, or IV M1a disease, as well as among patients who were treatment naïve. The most common side effects were fatigue, chills, fever, nausea, flu-like symptoms and pain at the injection site. IMLYGIC® should not be given to individuals with suppressed immune systems or who are pregnant because IMLYGIC® is a modified live oncolytic herpes virus therapy and herpes virus infection can potentially occur.
The authors concluded that IMLYGIC® is the first oncolytic immunotherapy to demonstrate therapeutic benefit in patients with advanced unresectable melanoma and may be another novel therapeutic option for patients with metastatic melanoma. Combining IMLYGIC® with T-Cell checkpoint inhibitor, YERVOY® (Ipilimumab) is presently being explored and thus far has shown encouraging results with minimal added toxicities. Talimogene Laherparepvec Improves Durable Response Rate in Patients With Advanced Melanoma. Andtbacka RH, Kaufman HL, Collichio F, et al. Published online before print May 26, 2015, doi: 10.1200/JCO.2014.58.3377

