There is an approximately 30% increased risk of breast cancer recurrence or death in those who are obese, compared to those with ideal body weight. Obesity is associated with alterations in Insulin/glucose homeostasis, adipokines, and sex hormones, which may all play a role in breast cancer outcomes.
BMI (Body Mass Index) does not discriminate between adiposity and muscle, and individuals deemed healthy based on a normal BMI may still be prone to cardiometabolic disorders due to high levels of visceral fat. It has been reported that approximately 18% of women with normal BMI had excess fat, detected on DEXA scan.
In a recently published article in JAMA Oncology involving 3460 postmenopausal women with normal BMI, there was a 56% increase in the risk of developing ER-positive breast cancer per 5-kg increase in trunk fat, despite a normal BMI. This study concluded that a normal BMI may not be an adequate proxy for the risk of breast cancer in postmenopausal women but high body fat levels and altered levels of circulating metabolic and inflammatory factors may be associated with a higher risk of invasive breast cancer.
High Body Fat Level Increases Breast Cancer Risk in Postmenopausal Women with Normal BMI
SUMMARY: Breast cancer is the most common cancer among women in the US and about 1 in 8 women (12%) will develop invasive breast cancer during their lifetime. Approximately 268,600 new cases of female breast cancer will be diagnosed in 2019 and about 41,760 women will die of the disease. Obesity is an important contributing factor to postmenopausal breast cancer incidence and mortality. Based on recently published meta-analysis, in women diagnosed with breast cancer, there is an approximately 30% increased risk of disease recurrence or death in those who are obese, compared to those with ideal body weight. Increasing physical activity may lower the risk of breast cancer recurrence. According to the consensus from the St Gallen Consensus Conference in 2015, obesity has been associated with poor breast cancer outcomes. Obesity is associated with alterations in Insulin/glucose homeostasis, adipokines, and sex hormones, which may all play a role in breast cancer outcomes. Weight loss can lead to reductions in C-reactive protein, Insulin, glucose, and Leptin. These mediators have all been implicated to have prognostic significance in breast cancer.
Body Mass Index (BMI) measures body size and is calculated based on height and weight. It is used as a measure of obesity. BMI however does not discriminate between adiposity and muscle and individuals deemed healthy based on a normal BMI may still be prone to cardiometabolic disorders due to high levels of visceral fat. Dual-energy X-ray absorptiometry (DXA or DEXA) is often utilized to measure bone mineral density and is the most accurate method presently available for the diagnosis of osteoporosis and to estimate fracture risk. DEXA scan can also be used to measure total body composition and fat content including the amount of visceral fat, with a high degree of accuracy by its ability to break down fat, bone and muscle tissue. It has been reported that approximately 18% of women with normal BMI had excess fat, detected on DEXA scan.
There are two types of adipose tissue in the human body, White Adipose Tissue (WAT) and Brown Adipose Tissue (BAT) which have antagonistic functions. White Adipose Tissue or white fat cells represent the body’s main type of fat tissue and each fat cell has a single lipid droplet. They are distributed in the subcutaneous tissue, around a person's waist and thighs and around internal organs (visceral fat). WAT stores excess energy as triglycerides and serves as an energy reservoir. Brown Adipose tissue (BAT) which is abundant in small mammals and in newborns generates heat by burning calories and helps them to survive cold temperatures. Brown adipocytes contain several small lipid droplets, and a high number of iron-containing mitochondria which gives brown fat its dark tan color. Most BAT is distributed in the lower neck and interscapular area of an adult, and above the collarbone. Higher quantities of BAT are associated with lower body weight and BAT decreases and body weight increases with increasing age.
Leptin is a hormone produced primarily by adipose tissue and circulating Leptin levels correlate with the body fat stores, with increased circulating Leptin levels noted in individuals with excess adiposity. Leptin can induce Aromatase which synthesizes estrogen, can directly stimulate cancer cell proliferation and survival, and activate Estrogen Receptor α via ligand-independent mechanism.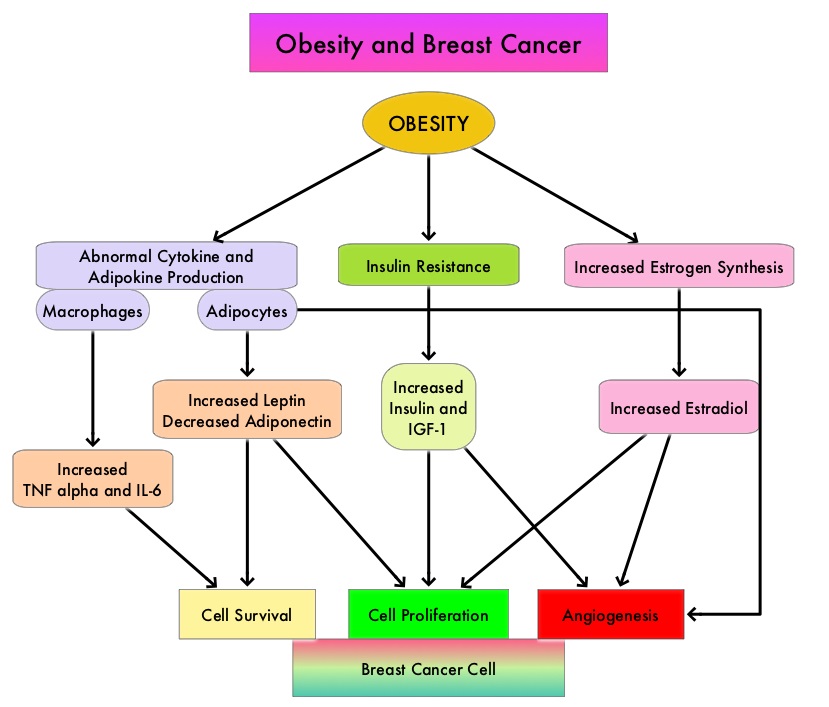
The increased risk of postmenopausal breast cancer in women with normal BMI is poorly understood. Recent studies have shown that in these women with normal BMI, excess body fat is associated with adipocyte hypertrophy which correlates with WAT inflammation, increased circulating Leptin levels, elevated levels of Aromatase and elevated Insulin levels. Dysregulation of Insulin signaling can activate the PI3K/Akt/mTOR and Ras/Raf/MAPK pathways which in turn can enhance cell proliferation and increase the risk of breast cancer. Further, Insulin also induces insulin like growth factor-1 (IGF-1), which can activate ERα. Insulin resistance leads to reduced levels of sex hormone–binding globulin, resulting in elevated levels of free estradiol. It has been suggested that all of these changes collectively may play a role in the pathogenesis of obesity-related breast cancer. The present study was conducted to investigate the association between body fat and breast cancer risk in women with normal BMI.
The authors in this long-term prospective study examined the association between body fat mass, measured by DEXA scan, and the risk of breast cancer, in a secondary analysis of 3460 postmenopausal women with normal BMI (18.5-24.9), enrolled in the Women’s Health Initiative (WHI) clinical trials or observational study. The goal of this study was to understand whether excess adipose tissue is associated with an increased breast cancer risk in women with normal BMI. Participants 50-79 years old with a mean age of 64 years underwent body fat measurement (the percentage of whole-body fat, trunk fat, and fat mass in both legs) with DEXA scan at 3 US designated centers at the time of study entry into the WHI clinical trials, and years 1, 3, 6, and 9. Levels of Insulin, glucose, C-reactive protein, interleukin-6, triglycerides, HDL cholesterol, estradiol, sex hormone-binding globulin, adiponectin, and Leptin were measured in 3-13% of participants using baseline fasting blood specimens.
At a median follow up of 16 years, 182 incident breast cancers were confirmed, and 146 (80%) were ER positive. It was noted that among postmenopausal women with normal BMI, relatively high body fat levels were associated with an elevated risk of invasive breast cancer. The authors specifically, found a 56% increase in the risk of developing ER-positive breast cancer per 5-kg increase in trunk fat, despite a normal BMI. Elevated trunk fat levels were also associated with metabolic dysregulation and inflammation characterized by increased circulating levels of Insulin, Leptin, C-reactive protein, Interleukin 6 and triglycerides, whereas levels of HDL cholesterol and sex hormone–binding globulin were lower.
It was concluded from this large prospective study that normal BMI may not be an adequate proxy for the risk of breast cancer in postmenopausal women. High body fat levels and altered levels of circulating metabolic and inflammatory factors may be associated with a higher risk of invasive breast cancer. Association of Body Fat and Risk of Breast Cancer in Postmenopausal Women with Normal Body Mass Index. A Secondary Analysis of a Randomized Clinical Trial and Observational Study. Iyengar NM, Arthur R, Manson JE, et al. JAMA Oncol. 2019;5:155-163

