SUMMARY: The American Cancer Society estimates that in 2016, about 72,580 people will be diagnosed with Non Hodgkin Lymphoma (NHL) in the United States and about 20,150 individuals will die of this disease. Indolent Non Hodgkin Lymphomas are mature B cell lymphoproliferative disorders and include Follicular Lymphoma, Nodal Marginal Zone Lymphoma (NMZL), Extranodal Marginal Zone Lymphoma (ENMZL) of Mucosa-Associated Lymphoid Tissue (MALT) lymphoma, Splenic Marginal Zone Lymphoma (SMZL), LymphoPlasmacytic Lymphoma (LPL) and Small Lymphocytic Lymphoma (SLL). Follicular Lymphoma is the most indolent form and second most common form of all NHLs and they are a heterogeneous group of lymphoproliferative malignancies. Approximately 20% of all NHLs are Follicular Lymphomas. Advanced stage indolent NHL are not curable and as such, prolonging Progression Free Survival (PFS) and Overall Survival (OS), while maintaining Quality of Life (QoL), has been the goals of treatment intervention. Asymptomatic patients with indolent NHL are generally considered candidates for “watch and wait” approach, whereas those with B symptoms (fever, night sweats, and weight loss), painful lymphadenopathy/splenomegaly, organ compromise and cytopenias are generally considered candidates for therapy.
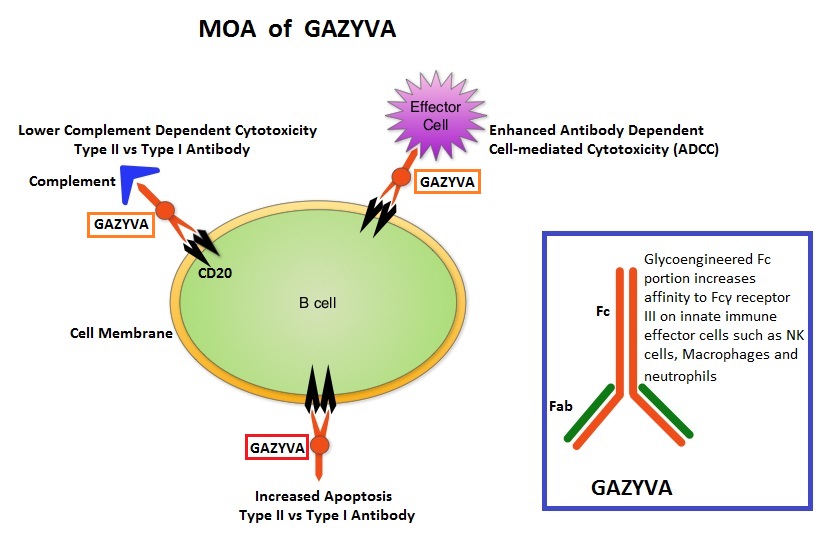 GAZYVA® (Obinutuzumab) is glycoengineered, fully humanized, third generation, type II anti-CD20 antibody (IgG1 monoclonal antibody) that selectivity binds to the extracellular domain of the CD20 antigen on malignant human B cells. By virtue of binding affinity of the glycoengineered Fc portion of GAZYVA® to Fcγ receptor III on innate immune effector cells such as natural killer cells, macrophages and neutrophils, Antibody-Dependent Cell-mediated Cytotoxicity (ADCC) and Antibody-Dependent Cellular phagocytosis is significantly enhanced, whereas it induces very little Complement-Dependent Cytotoxicity. This is in contrast to RITUXAN® (Rituximab), which is a first generation type I, chimeric anti-CD20 targeted monoclonal antibody that kills lymphoma cells primarily by Complement-Dependent Cytotoxicity and also ADCC.
GAZYVA® (Obinutuzumab) is glycoengineered, fully humanized, third generation, type II anti-CD20 antibody (IgG1 monoclonal antibody) that selectivity binds to the extracellular domain of the CD20 antigen on malignant human B cells. By virtue of binding affinity of the glycoengineered Fc portion of GAZYVA® to Fcγ receptor III on innate immune effector cells such as natural killer cells, macrophages and neutrophils, Antibody-Dependent Cell-mediated Cytotoxicity (ADCC) and Antibody-Dependent Cellular phagocytosis is significantly enhanced, whereas it induces very little Complement-Dependent Cytotoxicity. This is in contrast to RITUXAN® (Rituximab), which is a first generation type I, chimeric anti-CD20 targeted monoclonal antibody that kills lymphoma cells primarily by Complement-Dependent Cytotoxicity and also ADCC.
GADOLIN is a pivotal multicenter, open-label, phase III study in which TREANDA® (Bendamustine) alone was compared with TREANDA® plus GAZYVA® followed by GAZYVA® maintenance, in patients with indolent NHL (iNHL), refractory to RITUXAN®. Patients with RITUXAN® refractory iNHL (N=396) were randomly assigned to receive either TREANDA® 120 mg/m2 IV on days 1 and 2 every 28 days for a total of 6 cycles (N=202) or TREANDA® 90 mg/m2 IV on days 1 and 2 every 28 days for 6 cycles and GAZYVA® 1000mg IV days 1, 8 and 15 every 28 days of cycle 1 and on day 1 of cycles 2-6 (N=194). In patients with non-progressive disease in the combination arm, GAZYVA® was continued (maintenance) every 2 months for up to 2 years. Both treatment groups were well balanced and the median age was 63 years, with a median of two prior lines of therapy. More than 90% of patients in each treatment group were refractory to their previous therapy and between 76% and 81% were double-refractory to both RITUXAN® and an alkylating agent. The Primary end point was Progression Free Survival (PFS) and Secondary end points included Overall Survival and Response Rate.
The study was unblinded at the time of planned interim analysis and had to be halted early, upon recommendations from the Independent Data Monitoring Committee, as the primary end point was reached. After a median follow up of 21 months, the Progression Free Survival with GAZYVA® and TREANDA® combination was superior (median not reached) compared to a PFS of 14.9 months with TREANDA® monotherapy (HR=0.55; P<0.0001). This meant a 45% reduction in the rate of disease progression. There was however no difference in the Response Rates between the treatment groups and the best Overall Response Rate up to 12 months from start of treatment, was 77% in the TREANDA® alone group and 79% in the TREANDA® plus GAZYVA® group. Median Overall Survival has not yet been reached in either arm and longer follow up is needed. The combination experimental group experienced more grade 3 adverse events such as infusion related reactions and neutropenia whereas the TREANDA® alone group experienced more thrombocytopenia and anemia.
The authors concluded that GAZYVA® in combination with TREANDA® is superior to TREANDA® alone, in patients with RITUXAN® refractory indolent Non Hodgkin Lymphoma, with a significant improvement in Progression Free survival. The lack of difference in the Response Rate between the two treatment groups begs the question if the improvement in PFS benefit in the combination treatment group was derived from the quality of remission related to improved minimal residual disease negativity versus continuous maintenance treatment with GAZYVA® or both. Obinutuzumab plus bendamustine versus bendamustine monotherapy in patients with rituximab-refractory indolent non-Hodgkin lymphoma (GADOLIN): a randomised, controlled, open-label, multicentre, phase 3 trial. Sehn LH, Chua N, Mayer J, et al. Lancet Oncol 2016;17:1081-1093

