SUMMARY: The FDA on May 2, 2019, approved TIBSOVO® (Ivosidenib) for newly-diagnosed acute myeloid leukemia (AML) with a susceptible IDH1 (Isocitrate DeHydrogenase-1) mutation, as detected by an FDA-approved test, in patients who are at least 75 years old or who have comorbidities that preclude the use of intensive induction chemotherapy. The American Cancer Society estimates that for 2019, 21,450 new cases of Acute Myeloid Leukemia (AML) will be diagnosed in the United States and 10,920 patients will die of the disease. AML can be considered as a group of heterogeneous diseases with different clinical behavior and outcomes. Cytogenetic analysis has been part of routine evaluation when caring for patients with AML. By predicting resistance to therapy, tumor cytogenetics will stratify patients, based on risk and help manage them accordingly. Even though cytotoxic chemotherapy may lead to long term remission and cure in a minority of patients with favorable cytogenetics, patients with high risk features such as unfavorable cytogenetics, molecular abnormalities, prior Myelodysplasia and advanced age, have poor outcomes with conventional chemotherapy alone.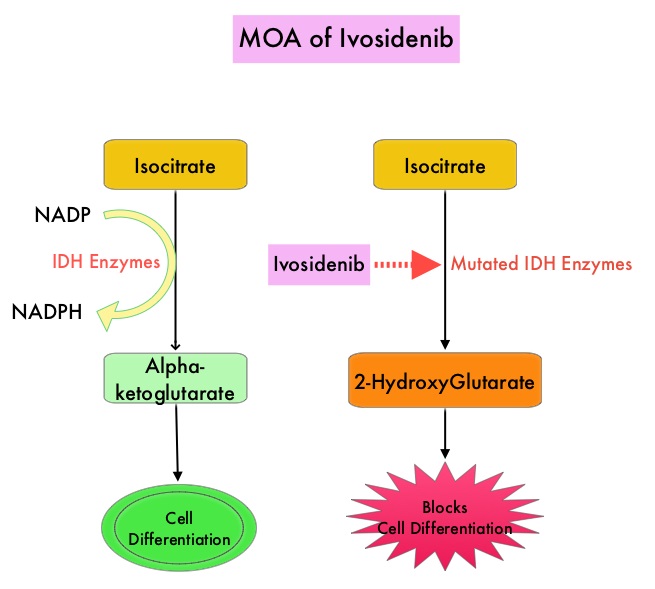
Isocitrate DeHydrogenase (IDH) is a metabolic enzyme that helps generate energy from glucose and other metabolites, by catalyzing the conversion of Isocitrate to Alpha-Ketoglutarate. Alpha-ketoglutarate is required to properly regulate DNA and histone methylation, which in turn is important for gene expression and cellular differentiation. IDH mutations lead to aberrant DNA methylation and altered gene expression thereby preventing cellular differentiation, with resulting immature undifferentiated cells. IDH mutations can thus promote leukemogenesis in Acute Myeloid Leukemia and tumorigenesis in solid tumors and can result in inferior outcomes. There are three isoforms of IDH. IDH1 is mainly found in the cytoplasm, as well as in peroxisomes, whereas IDH2 and IDH3 are found in the mitochondria, and are a part of the Krebs cycle. Approximately 20% of patients with AML, 70% of patients with Low-grade Glioma and secondary Glioblastoma, 50% of patients with Chondrosarcoma, 20% of patients with Intrahepatic cholangiocarcinoma, 30% of patients with Angioimmunoblastic T-cell lymphoma and 8% of patients with Myelodysplastic syndromes/Myeloproliferative neoplasms, are associated with IDH mutations. TIBSOVO® is an oral, targeted, small-molecule inhibitor of mutant IDH1. The FDA in July, 2018, approved TIBSOVO® for adult patients with relapsed or refractory AML with a susceptible IDH1 mutation.
The present first line approval by the FDA was based on an open-label, single-arm, multicenter clinical trial (Study AG120-C-001, NCT02074839)of single-agent TIBSOVO®, for newly-diagnosed AML patients, with an IDH1 mutation detected by an FDA-approved IDH1 Assay. In this study, 28 patients were included and these patients were at least 75 years old, or had comorbidities that precluded the use of intensive induction chemotherapy. For comorbidities, enrolled patients met at least one of the following criteria – baseline ECOG PS of 2 or more, severe cardiac or pulmonary disease, hepatic impairment with Bilirubin more than 1.5 times the upper limit of normal, or Creatinine Clearance less than 45 mL/min. The median age was 77 years and 79% had therapy-related AML or AML with Myelodysplasia-related changes. Patients received TIBSOVO® 500 mg orally daily until disease progression, development of unacceptable toxicity, or hematopoietic stem cell transplantation. Efficacy was based on the rate of Complete Remission (CR) or Complete Remission with partial hematologic improvement (CRh) rate, the duration of CR+CRh, and the conversion rate from transfusion dependence to transfusion independence. CRh was defined as less than 5% of blasts in the bone marrow, no evidence of disease, and partial recovery of peripheral blood counts (platelets more than 50,000/microliter and ANC more than 500/microliter).
In this trial, TIBSOVO® demonstrated a CR+CRh rate of 42.9%, with a CR rate of 28.6% and a CRh rate was 14.3%. The median durations of CR and CR+CRh could not be estimated, with 41.7% of those who achieved CR or CRh remaining on TIBSOVO® treatment as of the data cutoff (treatment duration ranged from 20.3 to 40.9 months). At 12 months after receiving treatment, 58.3% of patients who achieved CR or CRh, remained in remission. For those who achieved a CR or CRh, the median time to best response of CR or CRh was 2.8 months. Among those patients who were dependent on RBC and/or platelet transfusions at baseline, 41.2% achieved transfusion independence lasting at least 8 weeks.
The most common adverse reactions were fatigue, nausea, diarrhea, rash, pyrexia, arthralgia, leukocytosis and QT prolongation. One important side effect of the IDH inhibitors is the induction of differentiation of the malignant cells, and in 10-20% of patients, a clinical syndrome known as the IDH differentiation syndrome can occur. The IDH differentiation syndrome should be promptly managed by dose interruption and treatment with glucocorticoids, oral hydroxyurea, or both.
It was concluded that TIBSOVO® can induce durable responses among newly diagnosed poor risk AML patients with an IDH1 mutation, who are ineligible for intensive chemotherapy, fulfilling an unmet need. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-ivosidenib-first-line-treatment-aml-idh1-mutation

