SUMMARY: The FDA on June 22, 2017, granted regular approvals to TAFINLAR® (Dabrafenib) and MEKINIST® (Trametinib) administered in combination, for patients with metastatic Non Small Cell Lung Cancer (NSCLC), with BRAF V600E mutation, as detected by an FDA-approved test. These are the first FDA approvals specifically for treatment of patients with BRAF V600E mutation-positive metastatic NSCLC.
The FDA also approved the Oncomine® Dx Target Test, a next generation sequencing (NGS) test to detect multiple gene mutations for lung cancer in a single test from a single tissue specimen. This test detects the presence of BRAF, ROS1, and EGFR gene mutations or alterations in tumor tissue of patients with NSCLC. This test can be used to select patients with NSCLC with the BRAF V600E mutation for treatment with the combination of TAFINLAR® and MEKINIST®. This is the first NGS oncology panel test approved by the FDA for multiple companion diagnostic indications.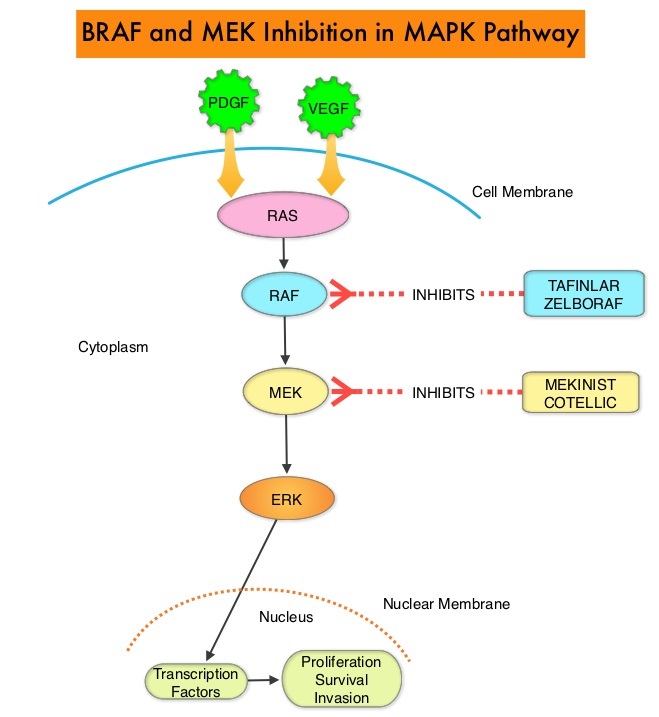
Combining MEKINIST® (Trametinib) with TAFINLAR® (Dabrafenib) to treat patients with NSCLC, was based on the understanding of the biological pathways of this malignancy. The Mitogen-Activated Protein Kinase pathway (MAPK pathway) is an important signaling pathway which enables the cell to respond to external stimuli. This pathway plays a dual role, regulating cytokine production and participating in cytokine dependent signaling cascade. The MAPK pathway of interest is the RAS-RAF-MEK-ERK pathway. This pathway has been implicated in the development of multiple malignancies including NSCLC and Melanoma. The RAF family of kinases includes ARAF, BRAF and CRAF signaling molecules. BRAF is a very important intermediary of the RAS-RAF-MEK-ERK pathway. BRAF mutations have been demonstrated in 6-8% of all malignancies. TAFINLAR® is a selective oral BRAF inhibitor and MEKINIST® is a potent and selective inhibitor of MEK gene, which is downstream from RAF in the MAPK pathway.
The approval of TAFINLAR® and MEKINIST® combination, for patients with metastatic NSCLC was based on an international, multicenter, three-cohort, non-randomized, open-label trial, in patients with locally confirmed BRAF V600E mutation-positive, metastatic NSCLC. In this phase II trial, 93 patients were treated with the combination of TAFINLAR® 150 mg orally twice daily and MEKINIST® 2 mg orally once daily. Of these 93 patients, 36 patients had received no prior systemic therapy for metastatic NSCLC and 57 patients received at least one prior platinum-based chemotherapy regimen and had disease progression. The third cohort in this phase II trial included 78 previously treated patients with BRAF V600E mutation-positive metastatic NSCLC, who received single-agent TAFINLAR®. The primary endpoint was Overall Response Rate (ORR).
It was noted that in the previously treated group, the ORR for the combination treatment based on independent review was 63% with a median Duration of Response of 12.6 months. In the treatment-naive group, the ORR for the combination was 61% and this group had not reached the endpoint for median Duration of Response and therefore was not estimable. However, among those who responded to treatment, 59% of the responders had response durations greater than 6 months. The ORR for patients who received single agent TAFINLAR® was 27% and the median Duration of Response was 9.9 months. The most common Grade 3-4 adverse reactions were pyrexia, fatigue, dyspnea, vomiting, rash, hemorrhage, and diarrhea.
It was concluded that TAFINLAR® plus MEKINIST® combination represents a new targeted therapy for patients with BRAF V600E mutation¬-positive metastatic NSCLC, who tend to respond less favorably to standard chemotherapy. This approval marks the fourth actionable genomic biomarker in metastatic NSCLC along with EGFR, ALK and ROS-1. Dabrafenib plus trametinib in patients with previously treated BRAF(V600E)-mutant metastatic non-small cell lung cancer: an open-label, multicentre phase 2 trial. Planchard D, Besse B, Groen HJ et al. Lancet Oncol. 2016 Jul;17(7):984-93. doi: 10.1016/S1470-2045(16)30146-2. Epub 2016 Jun 6.

